There is a black hole at the heart of biology,” Nick Lane writes at the start of his 2015 book The Vital Question. “Bluntly put, we do not know why life is the way it is.” It takes some chutzpah to lay down the gauntlet to the life sciences so starkly—to suggest they are engaged more in documenting life than in explaining it.
Lane, a professor of evolutionary biochemistry at University College London, doesn’t seem the kind of person you might expect to make such a provocative challenge. He’s disarmingly modest, explaining his own answer for why life is the way it is with enthusiasm and conviction but also with frequent disclaimers and frank recognition that he could be wrong. “I’ve come to realize that being wrong is actually really good fun,” he says.
But then his self-assurance kicks back in. Sure, he could be wrong on the details, but on the big picture, “I can’t possibly be, because at bottom all I am saying is that energy is important to life, and that the peculiar method by which it works surely tells us something important about how life operates.”
It takes chutzpah to lay down the gauntlet to the life sciences so starkly.
Lane and I are sitting in the Wellcome Collection in London, the flagship public space of the Wellcome Trust medical charity set up in 1936 from the legacy of Victorian pharmaceutical entrepreneur and collector Henry Wellcome. We are surrounded by Wellcome’s collection of bone saws, religious relics, and medical-themed paintings—an apt setting for talking about how life began, how it proceeds, and ends.
Lane displays the curious and perhaps even contradictory mindset that seems to characterize many creatively provocative scientists: open to being wrong, but convinced he is fundamentally right. He is eclectic in his sources of inspiration but focused on his personal vision. His independent turn of mind is reflected in his unconventional career path, which—along with the breadth of his vision—puts one in mind of the late James Lovelock, who also thought deeply about the relationship between life and the planetary environment it inhabits and transforms. And like Lovelock, Lane has the gift of being a clear communicator, which has allowed him to bring to wider attention a new perspective on living organisms that might otherwise have languished in the corners of specialist journals.
By asking questions about life that few others seem to be asking—and then offering ingenious answers—Lane has earned wide recognition, praise, and admiration. His 2010 book Life Ascending won the Royal Society Book Prize for science books, and in 2016 he was given the Royal Society’s prestigious Michael Faraday award for communication of science to the public. Bill Gates has said The Vital Question “blew me away”—and has funded Lane’s work to the tune of $1.2 million.

Through scientific and popular publications as well as lab experiments, Lane has been steadily developing an unconventional narrative about how life works and how it began and evolved. At its core, it is a tale not of genetic inheritance of information but of how life harnesses energy from its environment and uses it to build complex molecular systems. “The difference between being alive or dead,” he writes in his new book Transformer: The Deep Chemistry of Life and Death in an aphoristic summary of his central concept, “lies in energy flow.”
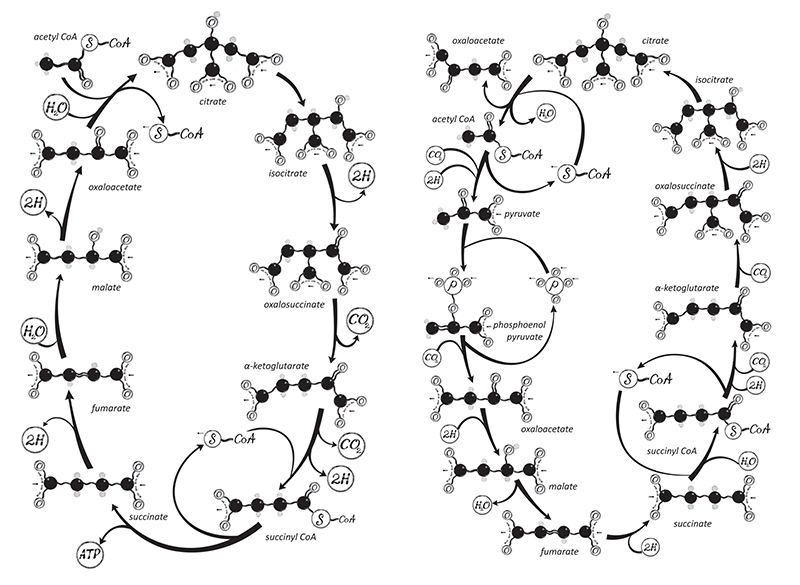
Transformer presents an explanation for the origin of the Krebs cycle, the central biochemical process of metabolism in all organisms that live by respiration, whereby large molecules are broken down into small ones and energy is generated in the process. Both prokaryotic organisms like bacteria and eukaryotes like us use the Krebs cycle, which was identified in 1937 by the British biochemist Hans Krebs (it is sometimes called the citric acid cycle). It generates cellular energy while producing the precursors of amino acids and other vital cell ingredients via a cyclic series of enzyme-catalyzed reactions.
The Krebs cycle is often seen as an important but extremely dry piece of textbook biochemistry. When I chatted to Lane a year ago at a science reception and he told me that this was the topic of his next book, he wore a wry grin that implied he was on a fool’s errand. “The Krebs cycle is basically extremely dull even to the people who work on it,” he tells me now. “And yet somehow it’s making me excited. I remember reading [the Italian chemist and writer] Primo Levi, who said a good writer should be able to make any subject interesting. And maybe I took that as a challenge.”
It’s no surprise that as gifted an expositor as Lane meets that challenge in style. The story in Transformer is not just lively and engaging but filled with stimulating ideas about life’s origins and evolution, about what regulates health and disease, and about the fundamental nature of life itself. “It’s about metabolic biochemistry, but also it’s about the origin of life, it’s about the origin of animals, it’s about cancer, it’s about consciousness,” Lane says. “These are really big ideas.”
In his position as leader of the largest group in UCL’s biology and biochemistry departments, Lane conducts experiments that seem closer to the inventive and speculative chemical cookery of the 1950s than to the cutting-edge wizardry of much of today’s molecular and chemical biology. The ingredients could hardly be simpler: carbon dioxide (CO2), hydrogen, iron minerals, salts. Out of them are coming, not exactly signs of life itself, but clues to how it began.
Lane got here along an unusual path. Having studied biochemistry at Imperial College London, he completed a Ph.D. at the Royal Free Hospital in London in the 1990s, which subsequently became part of UCL. “I got a good enough degree, but only just, and I had a big debt,” he says. “I got a job in a lab to pay off the debt, and it came with the opportunity to do a Ph.D.” The lab was run by a professor of surgery, Colin Green. Lane remembers Green as “an extraordinary man who set up the first medical school in Palestine and was director of the London City Ballet at the time.”
Lane worked on organ transplantation, trying to understand why transplants can lead to the condition called ischemia, where tissues are damaged by low levels of blood oxygen. A transplant can lead to a burst of free radicals in the body—reactive, oxidizing chemical agents with unpaired electrons. Lane tried introducing antioxidants to reduce the damage. “But nothing ever worked, and you think the dose is wrong or the timing is wrong or something,” he says. “In some ways, it was great fun and I realized that I love research. In other ways, it was frustrating.” Yet in retrospect, the essence of Lane’s grand vision of life was already contained in those failing experiments.
Lane’s bold hypothesis is life had to be this way.
Seeking a postdoctoral position in free-radical chemistry without knowing anyone in the field was never likely to be successful, but Lane found an alternative. “I had just been a runner-up in a newspaper writing competition, so I looked for writing jobs,” he explains. “Some of them were dreadful—writing up clinical trials reports for pharmaceutical companies, unbelievably dull.” He eventually landed a position working on medical animations. “It was basically soft marketing for pharmaceutical companies,” Lane says. “I did that for a couple of years, and I learned a lot about writing. First, tell a straight story. Second, use plain international English. And I found I could master a subject I knew little about; I could grasp the essence quickly. It turned out to be a useful skill.”
The job was only fun for so long. “My way out was that I’d try to write a book,” Lane says, as if it were the obvious option. In the 1990s, free-radical chemistry caused by oxygen-based respiration was thought to be central to many disease mechanisms. Why that is so was the organizing question for his first book, Oxygen: The Molecule That Made the World, published in 2002. “But I had no idea where the oxygen came from,” he says. “I didn’t know anything about the evolutionary history of life on Earth.” As he explored that question, “I kind of fell in love with science all over again.”
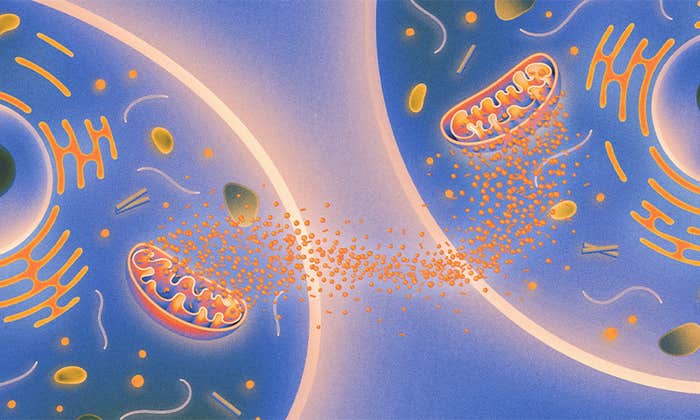
But he was left with a puzzling question. “Oxygen in evolutionary terms seems to have been a wonderful thing. The more oxygen in the atmosphere, the more profuse life has been. And yet here’s the medical profession saying too much oxygen is going to poison you with free radicals.” It all came down, he figured, to aerobic metabolism in the mitochondria: the compartments in cells where energy is generated in the form of the molecule ATP, via the Krebs cycle. That became the topic of his next book, Power, Sex, Suicide, published in 2005.
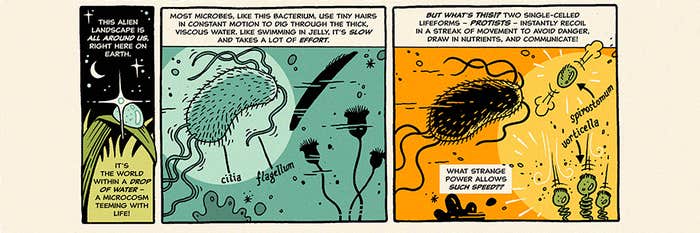
Mitochondria are widely believed now to be the remnants of an entirely distinct single-celled organism that merged with another cell long ago in evolutionary history, perhaps around 1.5 billion years ago, in a process called endosymbiosis. This would explain, for example, why our mitochondria today contain a small number of their own genes, separate from the rest of the genome in the cell nucleus. (It’s the possession of this nucleus that defines the distinction between cells like ours, called eukaryotic, and those of single-celled organisms such as bacteria and archaea, which are prokaryotes.)
There remains an argument, some of it heated, about what this momentous merger consisted of. Some researchers believe it happened between an already extant eukaryotic cell and a prokaryote. Others argue that both cells were prokaryotic. Lane favors the latter, saying those who advocate for the “eukaryote-first” picture do so for “basically emotional reasons.” He says the idea that mitochondria appeared when a bacterial endosymbiont merged with an archaeal host cell is consistent with all we know about the evolution of eukaryotes.
At any rate, cells that acquired their own internal powerhouse were suddenly able to do entirely new things—like group together to make multicellular organisms. Lane sharpened his ideas in conversations with the microbiologist Bill Martin of the University of Düsseldorf in Germany. Martin had an unconventional view of what happened in the endosymbiosis that led to eukaryotes with mitochondria, in which a central role was played not by oxygen but by hydrogen. In this “hydrogen hypothesis,” the host cell was an archaeon that metabolized by using hydrogen, and the bacterium that became the mitochondria produced hydrogen (as well as carbon dioxide) via anaerobic respiration—so it supplied the fuel that the host needed.
“Around 2002, when Oxygen came out, I went to a discussion meeting at the Royal Society and Bill was there and gave a pretty amazing talk on the origin of life,” Lane says. There Martin laid out the hydrogen hypothesis and explained why in his view early life was much more about metabolism and energy flow than about genes and proteins. “It was all very radical, it still is radical, but back then it seemed crazy,” Lane says. Martin, he adds, “was one of the biggest influences on my thinking.”
Stimulated by such ideas, Lane found that the book he was developing, Power, Sex, Suicide, started to take a new direction. “It went from being a book about mitochondria to being a book about the origin of complexity,” he says. “It really boiled down to the fact that it wasn’t about oxygen after all—it was about mitochondria and the energetics of genomes. I still think it’s basically the right way of seeing the question.”
Later, Lane and Martin teamed up, and in 2010 they made the case that what changed with the acquisition of mitochondria was the “energy per gene.” By their calculations, eukaryotes have up to 200,000 times more energy per gene than prokaryotes. With those resources, eukaryotes could do things unavailable to prokaryotes, such as become multicellular and develop complex forms and collective behavior. Thanks to that energy boost, large animals—and eventually us—became possible, as did behaviors, such as sexual reproduction, that are inaccessible to bacteria and archaea.
With these ideas about the energetics of life’s origins arising in the mid-2000s, Lane realized he was at a turning point. He was doing fine with science writing. He was embarked on writing Life Ascending, which looked at “the ten great inventions of evolution” (including the endosymbiosis that he believes led to eukaryotes). But he had, almost by accident, found a hypothesis about a key stage in evolutionary history, with a welter of wide-ranging implications.
The question of how life began is often posed in terms of how to make its molecular ingredients, such as amino acids and nucleotides, the building blocks of proteins and the nucleic acids RNA and DNA, from simple inorganic compounds that might have been present on the early Earth—carbon monoxide, water, and cyanide. If you succeed in making, say, nucleotides in such prebiotic reactions, the next challenge is to link them up into chains and polymers—for example, to progress toward the “RNA World” hypothesized in the 1980s, where RNA molecules served the dual role of acting as catalysts and carriers of inheritable “genetic” information before proteins and DNA took over those respective roles.
“It’s so simple. It solves so many problems. It’s quite thrilling.”
That’s all very well, says Lane, but to make such molecules and to do anything with them, you need a source of energy. In early work on prebiotic chemistry, like that of Harold Urey and Stanley Miller, the energy source driving the chemical reactions was often assumed to be light or ultraviolet radiation from the sun, or lightning discharges in the atmosphere. But pretty much all the energy in living cells today, whether of bacteria or plants or animals, is ultimately “chemiosmotic”: The cell’s ubiquitous molecule, ATP, produces energy by harnessing the driving force created by differences in concentration of ions—especially hydrogen ions, which are merely lone protons—across biological membranes. Like dam water driving a hydroelectric turbine, this “proton-motive force” powers the enzyme ATP synthase, a protein that sits embedded in the membrane and churns out ATP molecules as its head rotates in a membrane-bound sleeve. ATP synthase is found in almost all bacteria, archaea, and eukaryotes, says Lane. “It is as universal as the genetic code itself,” Lane writes in The Vital Question, and “should be as symbolic of life as the double helix of DNA.” In other words, life is really all about the flow of electrical charge (electrons and protons) as a driving force for making biomolecules and powering metabolic pathways.
“I was actively trying to think, how can I test some of these ideas?” he recalls. “How can I get back into academia?” Then he got a lucky break.
In 2008, Lane met the author and scientist Don Braben, an advocate of “blue-skies research” that is curiosity-led rather than aimed at specific questions or applications. Braben was working at UCL, where he now holds an honorary position in the Office of the Vice-Provost for Research. “Don’s view is that some of the great breakthroughs in the 20th century would never have happened with the current funding model,” Lane says. Braben persuaded the provost at UCL to back research proposals with basically no peer review. “He wanted very short proposals on ideas that were potentially transformative,” Lane says. “It became clear that this was by far my best opportunity to get back into academia.”
After much back-and-forth, Lane’s application elicited a funded position called the Provost’s Venture Research Fellowship from 2009 until 2012. “It was strange for me to go back into the lab,” he says. “It was incredibly cack-handed and amateurish, and I didn’t have any equipment—I had to beg and borrow other people’s. I was doing amazingly stupid things, but some of it worked.”
Lane’s proposal was titled Chemiosmosis and the foundations of complex life. “It only took one or two meetings for me to be convinced that Nick is an exceptional scientist who might one day win a Nobel Prize,” says Braben. “He did not try to persuade me that his research would lead to this or that wonderful outcome but would concentrate entirely on the science involved.”
“Nick’s performance at our meetings convinced me that here was a scientist who would do something important and radically change the way we think,” Braben adds. “But with funding success rates running at about 20 percent, the chances of his winning support from normal sources would be negligible.”
Lane praises UCL as “an environment where people do give a damn about ideas and about other people’s work. It’s a really bottom-up, collaborative place, where you can easily infect people with your own enthusiasm.”
In The Vital Question, Lane offered a detailed and concrete scenario for how life might have got started on Earth. It builds on the idea proposed in the 1970s that it all began not in some prebiotic soup such as ponds or shallow oceans rich in organic molecules and warmed by sunlight, but deep down in the oceans at structures called hydrothermal vents, where volcanic activity sends mineral-rich hot fluids spewing from mineralized chimney-like structures on the seabed.
In the late 1980s, British chemist Mike Russell began to develop a detailed scenario for how the necessary chemistry could have arisen. Iron sulfide minerals might act as the source of the energy and electrons needed to drive the fixation of carbon, he said. Some vents are rich in such minerals, which can drive “redox chemistry” (for example, adding and subtracting oxygen and hydrogen atoms from carbon compounds) as the iron ions cycle between two states with differing electrical charge. Even today, clusters of iron and sulfur atoms are commonly found in some enzymes, including those that catalyze the reactions of respiration—a remnant, Russell suspected, of the early role played by iron sulfide minerals.
These things are where happiness in science lies.
This picture, called the chemoautotrophic origin of life, drew on work in the 1980s by the German chemist Günter Wächtershäuser, who argued that the Krebs cycle, running in reverse, could have been the first biochemical metabolic pathway, powered by iron-sulfur chemistry. In 1997 Russell and his colleague Allan Hall showed how the gradients in pH at alkaline vents could couple to iron sulfide redox chemistry to drive the emergence of the first living entities, which used a proton-motive force to power their metabolism.
To harness such a “chemiosmotic” concentration gradient, you need a membrane that partitions space into regions of different concentration, so that this difference is not just washed away by diffusion of the molecules or ions. At hydrothermal vents, Russell argued, such membranes can be inorganic—made from mineral compounds such as iron sulfides or hydroxides. Perhaps once such structures were producing pseudo-metabolic chemical processes that generated molecules like organic fatty acids, they could create their own organic membranes, the precursors to the phospholipid membranes of today’s cells. A system like this could act to transfer electrons from hydrogen molecules to carbon dioxide molecules (both are abundant in hydrothermal vents): the first step in generating more complex organic molecules such as formaldehyde, carboxylic acids, and ultimately the components of the Krebs cycle of metabolism.
In other words, rather than finding separate routes for making the biomolecular components of the first proto-cellular organisms and then having to hope they somehow all come together in an organized entity, redox chemistry at hydrothermal vents could have given rise to compartmentalized chemical reactors with a ready-made energy source, spinning out the complex organic ingredients needed for life to begin.
Russell’s scenario in which proton gradients and the proton-motive force operate in cell-like cavities at alkaline hydrothermal vents “really excited me,” says Lane. “Mike Russell was and is inspirational in linking geochemistry and structure with the beginnings of biochemistry.”
“An environment like hydrothermal events provide everything we think we need” to kickstart life, Lane says: the raw ingredients such as a carbon source (CO2), a source of energy (pH gradients), agents that can catalyze redox chemistry (such as iron sulfides), and compartmentalization by membranes.
Indeed, in the mid 2000s Martin and Russell elaborated the theory of an origin of life at alkaline hydrothermal vents and how the inorganic chemistry happening there could have given rise to the beginnings of the Krebs cycle that fixes carbon from CO2 into a form usable by early organisms. They developed a scenario in which these proto-organisms might have developed first into simple bacteria-like cells with an organic membrane, and then, about 2 billion years ago, merged to form eukaryotes with mitochondria.
Work by Martin and Russell is a foundation of Lane’s theory of the origin of life. Broadly speaking, Russell says, “Nick seems to have followed me in most of his ideas, although with a more biochemical grounding compared to mine, which was developed from geological exploration and geochemical knowledge and experience.” Martin is less sanguine. He says he has not been given enough credit for initiating some of the ideas described in Lane’s books (even though he is duly cited many times). Russell says he harbors no ill feelings about the way Lane has extended and popularized his ideas. “I, for one, am very happy about the way he proselytizes this work,” he says.
As Lane explains his vision in the gloom of the Wellcome exhibition room, while the occasional visitor wanders past, peering into the glass cases, I can see how convinced he is that this story, despite its chemical complexity, provides a coherent picture of the way life gained a foothold on the planet. Putting chemiosmotic energy and metabolic reactions at the heart of it all, he says, fits nicely with what genetic studies suggest the first organisms might have looked like. And his hypothesis “makes a terrifying prediction,” he says. “All of this biochemistry is just going to spontaneously happen. But what a beautiful prediction!” Now he’s trying to test it in the lab.
Transformer fleshes out this story by explaining how it could have led to the Krebs cycle itself. As Lane writes, this complex, cyclical series of reactions generates ATP “by stripping out hydrogen atoms from the carbon skeletons of food [molecules] and feeding them to the ravenous beast that is oxygen”: the process of cellular respiration. On paper it looks like a rather arbitrary sequence of conversions of molecules related to citric acid—a headache for biochemistry students to learn and understand. But Lane now believes that a consideration of what is needed to drive metabolism involving CO2, oxygen, and hydrogen makes almost all this chemistry inevitable.
Five or six years ago, Lane says, he’d have conceded only that something vaguely resembling this sequence was bound to arise. “It kind of beggars belief that that level of reaction networks will happen spontaneously. But it looks more and more like they do.” Lane is referring to not just the cycle itself but the reactions that spin off from it, which form amino acids and the specific nucleotide bases found in DNA and RNA. “If you’ve got this molecule here in this context, you ask, what’s going to happen next? And you can see what it will be. You are going to form this one, and so on. You start drawing these things, and you think—Jesus, here’s the Krebs cycle! You almost can’t end up with anything else.”
“It was all very radical, it still is radical, but back then it seemed crazy.”
If Lane is right, it suggests that identical biochemistry will arise on any other planet with an environment something like that of the prebiotic Earth. In other words, the principles of chemical thermodynamics and kinetics dictate a kind of pre-Darwinian convergent evolution that creates something at least close to a Krebs cycle. “All it’s asking for is a wet rocky planet or moon with CO2 in the atmosphere,” Lane says. “That’s not a big ask, right? There are probably billions of exoplanets in the Milky Way that fit that set of criteria.” And you need chemical disequilibrium like that found at hydrothermal vents. “What really matters is converting this environmental disequilibrium into living matter: CO2 and hydrogen into cycling intermediates, and amino acids and nucleotides.” The bold hypothesis is that life had to be this way—and not just terrestrial life, but any life in Earth-like environments. “It’s so simple,” Lane says. “It solves so many problems. It’s quite thrilling.”
Where evolution might take it after the first primitive protocells have appeared is another matter. So far Lane has elaborated his picture to account for the production of the first nucleotides and their polymers, and recently he and his group have argued that the energetics of CO2 fixation might explain why the genetic code, which relates the nucleotide sequence of DNA to the amino-acid sequence of proteins, takes the form it does. “I’ve just got up to the origins of information and evolvability,” Lane says, “but I’m a long way from molecular machines like ribosomes,” the multi-molecule assemblies that make proteins from the information in RNA. “So there’s an interesting next question: What’s the simplest possible molecular machine that you can imagine?”
But is there any proof that this is really how life began—and had to begin? “It makes a bunch of predictions that may or may not be true,” Lane admits. “It’s very easy to delude yourself into thinking that because it’s a beautiful idea, it’s got to be true. Maybe it’s not—maybe these associations are just not strong enough or there is some other explanation for it.”
The proposal needs to be tested in the lab—and that’s what Lane is now doing. He and his team are using microfluidic systems to see if, starting with CO2, hydrogen, and simple inorganic ingredients such as silicates and iron salts, they can generate some of the Krebs cycle compounds. They’re getting somewhere, slowly. “We’re getting at least acetate, and possibly pyruvate,” he says—two of the key molecules in the cycle. They have also shown that “protocells”—closed compartments made from organic membranes composed of simply fatty acids—will form spontaneously under the conditions that exist at alkaline hydrothermal vents.
“I seem to have assembled around me a bunch of people who are very free thinkers,” he says. “I encourage them to disprove everything that I say, and they seem to try and do it with relish. It throws lots of googlies at you on an almost daily basis: Either this is wrong or what can we do next, how can we get out of this corner?”
For all the grand ideas about some of the most profound questions contemplated by science, Lane hasn’t lost sight of his early days working on disease. That’s all part of the picture too. “The issue with cancer is it’s about growth,” he says. “It’s about how a cancer cell can optimize metabolic flux so that it can make copies of itself.” Cancer is also about the Krebs cycle. “The cancer people have been thinking along these lines for a little while, and it links up with aging as well. It’s hooking up three different fields in a way which they should hook up.”
An interest in this chemistry of carbon fixation has also come from another unexpected direction. “Part of the reason it’s become more interesting to people is because of the issues with CO2 capture and climate change,” Lane says. “People are now taking the idea that you can reduce CO2 and convert it into something useful like hydrocarbons much more seriously than they were a few years ago.”
Lane is willing to take his ideas about the centrality of energy and electron flow in some speculative directions. Transformer ends with a discussion of its role in the evolution of thought and consciousness. “The electrical potential humming away on cell membranes is movement too, dancing charge, electrons and protons, the elementary particles of life,” Lane writes. “Moving charge generates electromagnetic fields that permeate our being. And clearly, the flux of metabolism generates electromagnetic fields on cells. Could feelings somehow be related to this dance of charge, the ephemeral states of cells?”
“I think the likelihood of that being completely true is probably quite small,” Lane admits. “But the possibility that it might be true is intriguing.”
This seems to be the next direction in which Lane’s fertile mind is taking him. “If you’d asked me a couple of years ago which question I’d like to see answered in my lifetime, I’d almost certainly say something to do with the origin of life,” he says. “But in a strange way, I feel as if conceptually I can almost understand it now. There’s an awful lot of work in the lab to do, but I think I can join the dots. So now I’d say it’s consciousness, because I think that’s more out there.”
Despite being head of a large research group, formulating and testing complex hypotheses, Lane retains his passion—indeed, almost a sense of urgent obligation—for communicating science to the widest possible audience. “Asking the big questions and trying to excite other people with them—these things are where happiness in science lies,” Lane says. “Somehow we’ve got to get across to people that science is a human activity, that it’s about finding out what we don’t know about the world.”
In a time when popular science can end up over-simplifying ideas for general consumption, Lane’s books might seem uncompromising. Transformer is filled with illustrations of the Krebs-cycle intermediates and their reactions: yes, molecules, a pretty reliable deterrent for those with bad memories of school chemistry classes. “It’s going to require patience,” he admits. “It’s going to require some commitment on the part of the reader. But I’m not patronizing them. I’m giving them the opportunity to see why it’s this way.”
This urge to communicate without proselytizing or saying simply “Trust me, I’m a scientist,” is at the core of Lane’s mission. “What keeps most scientists going most of the time is the excitement of the unknown,” he says. “Yet somehow that’s not transmitted through to the public very well. If we can do that, I think there will be much more excitement about and sympathy for science in society, and then better decisions about what we do about climate emergencies or pandemics. It’s not just a case of getting science across to people, it’s a case of getting people to participate, to be part of it, to feel that science is for them.” ![]()
Philip Ball is a science writer and author based in London. His most recent book is The Book of Minds.
Lead image: Philipp Ammon