Steelers Pro Bowl center Mike Webster was never your typical meathead. During his heyday in the 1970s and 80s, he relied on his wits, as much as his brawn, to best opponents. “He was so smart, so prepared for everything we would face in a game,” his teammate, quarterback Terry Bradshaw, reflected in 2002. “I couldn’t have been the player I was without him.” Yet at age 50 Webster was living out of his truck, a garbage bag taped over the window. He died destitute, barely capable of remembering to eat.
While Webster’s mental decline and that of other prominent NFL players—Dave Duerson, Junior Seau—took years or decades to progress, the cellular origins of that decline have remained mysterious until recently. Studies on animals at the University of Pennsylvania suggest that misfolded tau proteins—the kind that often turn up post-head injury—are capable of spreading in the brain, triggering a quiet, steady march of neural deterioration. If confirmed in humans, this process could help explain why the player who gives perfectly lucid post-game interviews slides into dementia years after hanging up his helmet for good.
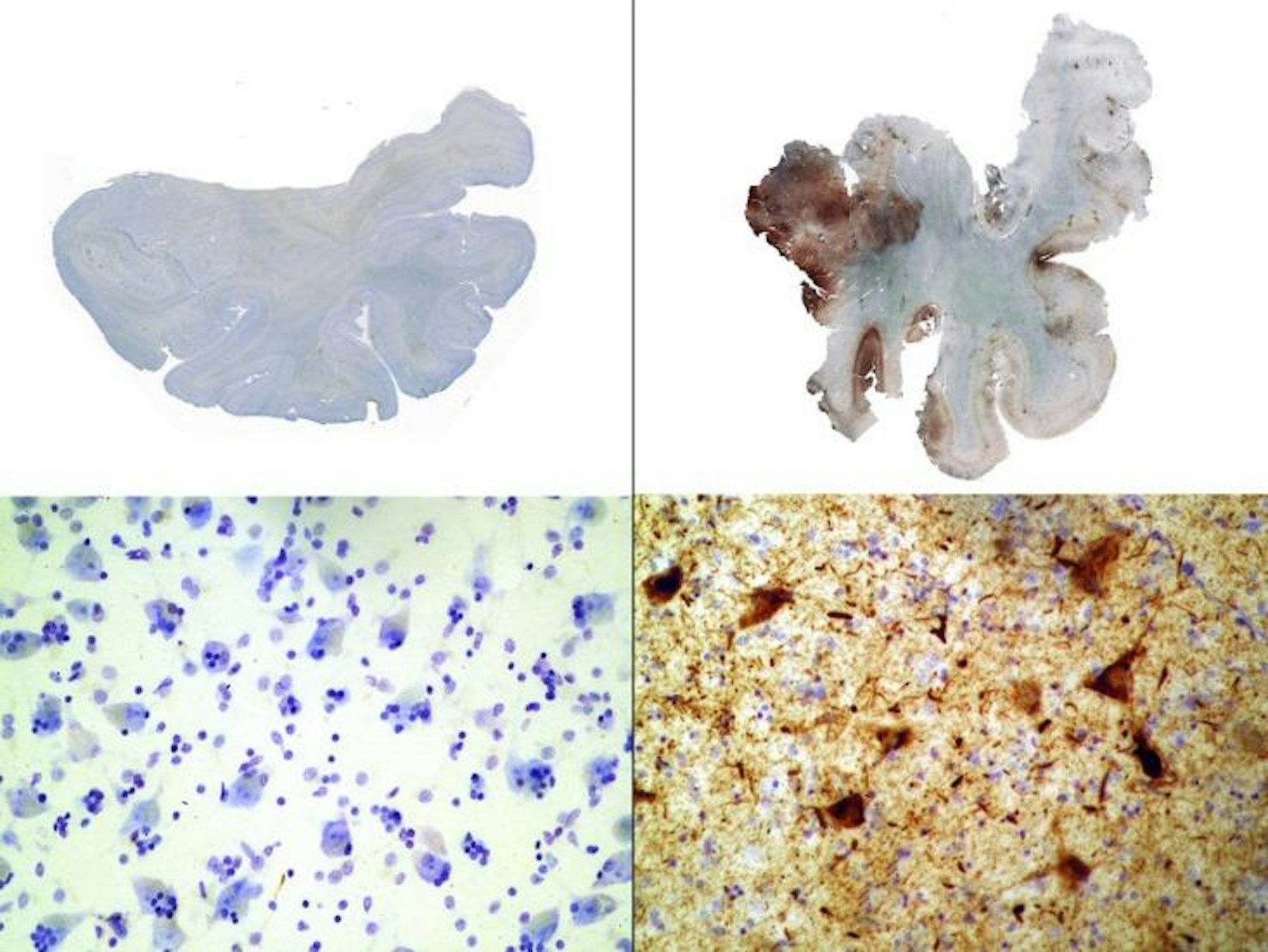
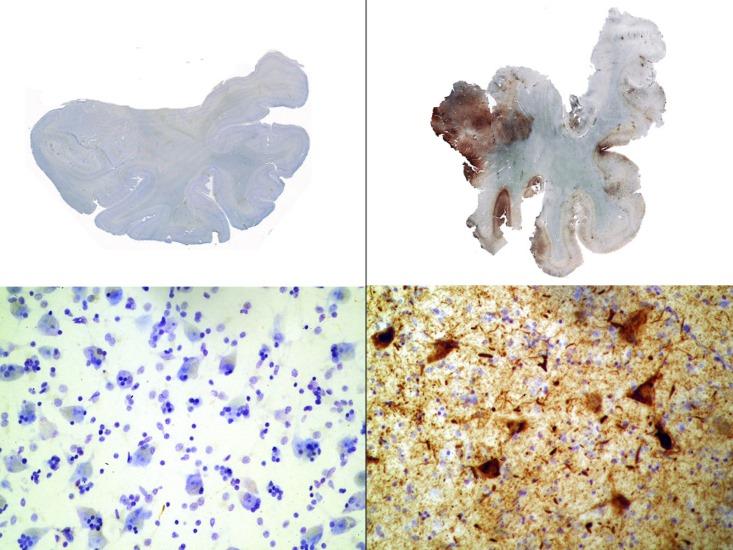
What happens in a football player’s brain after a hard hit to the head is still not completely understood, but here’s some of what we know: As a player crashes into his opponent, his brain sloshes around in the cerebrospinal fluid that surrounds it. This motion stretches long, thin brain cell fibers called axons out of shape, leading to a release of calcium into the cells. This influx of calcium, in turn, may activate enzymes that deform tau proteins inside the cells. “The tau becomes abnormally configured,” explained Boston University neuropathologist Ann McKee at February’s American Association for the Advancement of Science meeting. The protein can tangle, for example, or fold in on itself. If enough of the deadwood-like diseased tau builds up, it can block off pathways in the brain that carry cargo and essential nutrients, leading to cell death. Excess tau accumulation is one hallmark of chronic traumatic encephalopathy (CTE), the degenerative brain disease Webster, Duerson, and Seau were all found to have at autopsy.
But pathological tau buildup doesn’t necessarily end when players retire—it may actually take on a life of its own. In 2013, University of Pennsylvania biochemist Virginia Lee’s team reported that after they injected young mice’s brains with tiny fibrils of synthetic tau protein, abnormal tau gradually spread from one region of the brain to the next. The defective protein appears to propagate through terminals, the knob-like ends of axons that brain cells use to communicate with each other. “The terminals are picking up the bad protein and transmitting it back to the cell body [the central part of the neuron],” says Lee. “Eventually, you see that the cell body also has a tangle.” From there, the misfolded protein could travel to other healthy cells and corrupt them in the same way.
This destructive cascade is somewhat similar to that seen in disorders caused by prions, such as mad cow disease, in that both involve misfolded brain proteins. But prions, Lee points out, are infectious agents that cause swift brain cell demise, whereas there’s no evidence that tangled tau—also seen in conditions like Alzheimer’s—can be transmitted from one person to the next.
Lee suspects football players who absorb thousands of head hits each season may be vulnerable to ongoing, insidious cellular damage, especially if they sustain multiple larger injuries in a row. “You can imagine that a cell has increased tau as a reaction to traumatic brain injury, [and] maybe a small amount of this tau is not cleared and now [is] misfolded into abnormal seeds,” Lee says. Once these seeds are planted, they may spread and even feed off of future post-collision tau spikes. “If you have another injury a month later, that little seed could recruit the surge of tau to make a larger amount of pathology.”
Questions remain about just how gradual tau spread might contribute to the cognitive issues seen in athletes like Webster—and in the 6 percent of former NFL players over 50 who have a dementia-related diagnosis. Lee doesn’t know, for instance, if a certain level of repeated injury might touch off a cell-to-cell cascade of destruction, or if the threshold might be different in different people. Similarly, she says, diseased protein accumulation doesn’t always translate predictably into dementia. “Many normal individuals have tangles in their brain and yet they’re not impaired. We want to study what the protective factors are.”
If Lee and other researchers can pin down why only some former players develop significant mental impairment, they might be able to develop drug treatments to halt the molecular cascade that ruins players’ lives—or, even better, prevent it before it occurs.
Elizabeth Svoboda is a writer in San Jose, California, and the author of What Makes a Hero?: The Surprising Science of Selflessness.