On January 5, six days after China officially announced a spate of unusual pneumonia cases, a team of researchers at Shanghai’s Fudan University deposited the full genome sequence of the causal virus, SARS-CoV-2, into Genbank. A little more than three months later, 4,528 genomes of SARS-CoV-2 have been sequenced,1 and more than 883 COVID-related clinical trials2 for treatments and vaccines have been established. The speed with which these trials will deliver results is unknown—the delicate bаlance of efficacy and safety can only be pushed so far before the risks outweigh the benefits. For this reason, a long-term solution like vaccination may take years to come to market.3
The good news is that a lack of treatment doesn’t preclude an end to the ordeal. Viral outbreaks of Ebola and SARS, neither of which had readily available vaccines, petered out through the application of consistent public health strategies—testing, containment, and long-term behavioral adaptations. Today countries that have previously battled the 2002 SARS epidemic, like Taiwan, Hong Kong, and Singapore, have shown exemplary recovery rates from COVID. Tomorrow, countries with high fatality rates like Sweden, Belgium, and the United Kingdom will have the opportunity to demonstrate what they’ve learned when the next outbreak comes to their shores. And so will we.
The first Ebola case was identified in 1976,4 when a patient with hemorrhagic symptoms arrived at the Yambuku Mission Hospital, located in what is now the Democratic Republic of Congo (DRC). Patient samples were collected and sent to several European laboratories that specialized in rare viruses. Scientists, without sequencing technology, took about five weeks to identify the agent responsible for the illness as a new member of the highly pathogenic Filoviridae family.
The first Ebola outbreak sickened 686 individuals across the DRC and neighboring Sudan. 453 of the patients died, with a final case fatality rate (CFR)—the number of dead out of number of sickened—of 66 percent. Despite the lethality of the virus, sociocultural interventions, including lockdowns, contact-tracing, campaigns to change funeral rites, and restrictions on consumption of game meat all proved effective interventions in the long run.
That is, until 2014, when there was an exception to the pattern. Ebola appeared in Guinea, a small country in West Africa, whose population had never before been exposed to the virus. The closest epidemic had been in Gabon, 13 years before and 2,500 miles away. Over the course of two years, the infection spread from Guinea into Liberia and Sierra Leone, sickening more than 24,000 people and killing more than 10,000.
Countries that have previously battled the 2002 SARS epidemic, like Taiwan and Hong Kong, have shown exemplary recovery rates.
During the initial phase of the 2014 Ebola outbreak, rural communities were reluctant to cooperate with government directives for how to care for the sick and the dead. To help incentivize behavioral changes, sociocultural anthropologists like Mariane Ferme of the University of California, Berkeley, were brought in to advise the government. In a recent interview with Nautilus, Ferme indicated that strategies that allowed rural communities to remain involved with their loved ones increased cooperation. Villages located far from the capital, she said, were encouraged to “deputize someone to come to the hospital, to come to the burial, so they could come back to the community and tell the story of the body.” For communities that couldn’t afford to send someone to the capital, she saw public health officials adopt a savvy technological solution—tablets to record video messages that were carried between convalescent patients and their families.
However, there were also systemic failures that, in Ferme’s opinion, contributed to the severity of the 2014 West African epidemic. In Sierra Leone, she said, “the big mistake early on was to distribute [weakly causal] information about zoonotic transmission, even when it was obviously community transmission.” In other words, although there had been an instance of zoonotic transmission—the virus jumping from a bat to a human—that initiated the epidemic, the principle danger was other contagious individuals, not game meat. Eventually, under pressure from relief groups, the government changed its messaging to reflect scientific consensus.
But the retraction shook public faith in the government and bred resentment. The mismatch between messaging and reality mirrors the current pandemic. Since the COVID outbreak began, international and government health officials have issued mixed messages. Doubts initially surfaced about the certainty of the virus being capable of spreading from person to person, and the debate over the effectiveness of masks in preventing infection continues.
Despite the confused messaging, there has been general compliance with stay-at-home orders that has helped flatten the curve. Had the public been less trusting of government directives, the outcome could have been disastrous, as it was in Libera in 2014. After a two-week lockdown was announced, the Liberian army conducted house-to-house sweeps to check for the sick and collect the dead. “It was a draconian method that made people hide the sick and dead in their houses,” Ferme said. People feared their loved ones would be buried without the proper rites. A direct consequence was a staggering number of active cases, and an unknown extent of community transmission. But in the end, the benchmark for the end of Ebola and SARS was the same. The WHO declared victory when the rate of new cases slowed, then stopped. By the same measure, when an entire 14-day quarantine period passes with no new cases of COVID-19, it can be declared over.
It remains possible that even if we manage to end the epidemic, it will return again. Driven by novel zoonotic transmissions, Ebola has flared up every few years. Given the extent of COVID-19’s spread, and the potential for the kind of mutations that allow for re-infection, it may simply become endemic.
Two factors will play into the final outcome of COVID-19 are pathogenicity and virulence. Pathogenicity is the ability of an infectious agent to cause disease in the host, and is measured by R0—the number of new infections each patient can generate. Virulence, on the other hand, is the amount of harm the infectious agent can cause, and is best measured by CFR. While the pathogenicity of Ebola, SARS, and SARS-CoV-2 is on the same order—somewhere between 1 to 3 new infections for each patient, virulence differs greatly between the two SARS viruses and Ebola.
The case fatality rate for an Ebola infection is between 60 to 90 percent. The spread in CFR is due to differences in infection dynamics between strains. The underlying cause of the divergent virulence of Ebola and SARS is largely due to the tropism of the virus, meaning the cells that it attacks. The mechanism by which the Ebola virus gains entry into cells is not fully understood, but it has been shown the virus preferentially targets immune and epithelial cells.5 In other words, the virus first destroys the body’s ability to mount a defense, and then destroys the delicate tissues that line the vascular system. Patients bleed freely and most often succumb to low blood pressure that results from severe fluid loss. However, neither SARS nor SARS-CoV-2 attack the immune system directly. Instead, they enter lung epithelial cells through the ACE2 receptor, which ensures a lower CFR. What is interesting about these coronaviruses is that despite their similar modes of infection, they demonstrate a range of virulence: SARS had a final CFR of 10 percent, while SARS-CoV-2 has a pending CFR of 1.4 percent. Differences in virulence between the 2002 and 2019 SARS outbreaks could be attributed to varying levels of care between countries.
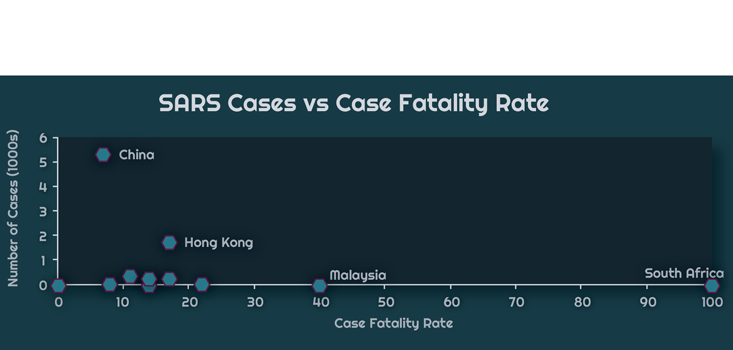
The chart above displays WHO data of the relationship between the total number of cases in a country and the CFR during the 2002-2003 SARS-CoV epidemic. South Africa, on the far right, had only a single case. The patient died, which resulted in a 100 percent CFR. China, on the other hand, had 5,327 cases and 349 deaths, giving a 7 percent CFR. The chart below zooms to the bottom left corner of the graph, so as to better resolve critically affected countries, those with a caseload of less than 1,000, but with a high CFR.
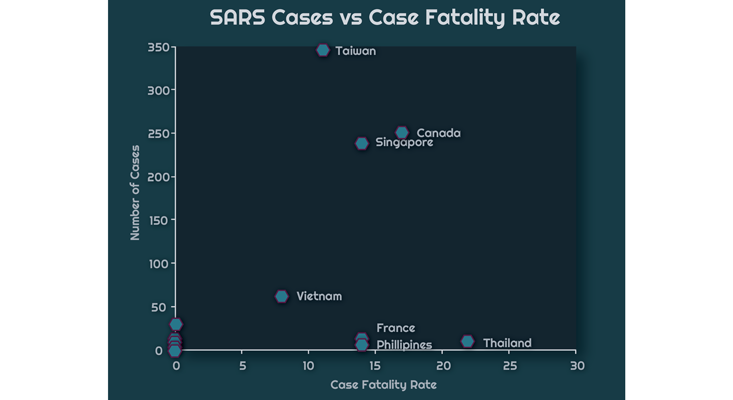
Here is Hong Kong, with 1,755 cases and a 17 percent CFR. There is also Taiwan, with 346 cases and an 11 percent CFR. Finally, nearly tied with Canada is Singapore with 238 cases and a 14 percent CFR.
With COVID-19, it’s apparent that outcome reflects experience. China has 82,747 cases of COVID, but has lowered their CFR to 4 percent. Hong Kong has 1,026 cases and a 0.4 percent CFR. Taiwan has 422 cases at 1.5 percent CFR, and Singapore with 8,014 cases, has a 0.13 percent CFR.
It was the novel coronavirus identification program established in China in the wake of the 2002 SARS epidemic that alerted authorities to SARS-CoV-2 back in November of 2019. The successful responses by Taiwan, Hong Kong, and Singapore can also be attributed to a residual familiarity with the dangers of an unknown virus, and the sorts of interventions that are necessary to prevent a crisis from spiraling out of control.
In West Africa, too, they seem to have learned the value of being prepared. When Ferme returned to Liberia on March 7, she encountered airport staff fully protected with gowns, head covers, face screens, masks, and gloves. By the time she left the country, 10 days later, she said, “Airline personnel were setting up social distancing lines, and [rural vendors] hawking face masks. Motorcycle taxis drivers, the people most at risk after healthcare workers—all had goggles and face masks.”
The sheer number of COVID-19 cases indicates the road to recovery will take some time. Each must be identified, quarantined, and all contacts traced and tested. Countries that failed to act swiftly, which allowed their case numbers to spiral out of control, will pay in lives and dollars. Northwestern University economists Martin Eichenbaum et al. modeled6 the cost of a yearlong shutdown to be $4.2 trillion, a cost that proactive countries will not face. A recent Harvard study7 published in Science suggests the virus will likely make seasonal appearances going forward, potentially requiring new waves of social distancing. In other words, initial hesitancy will have repercussions for years. In the future, smart containment principles,6 where restrictions are applied on the basis of health status, may temper the impact of these measures.
Countries that failed to act swiftly, which allowed their case numbers to spiral out of control, will pay in lives and dollars.
Inaction was initially framed as promoting herd immunity, where spread of the virus is interrupted once everyone has fallen sick with it. This is because getting the virus results in the same antibody production process as getting vaccinated—but doesn’t require the development of a vaccine. The Johns Hopkins Bloomberg School of Public Health estimates that 70 percent of the population will need to be infected with or vaccinated against the virus8 for herd immunity to work. Progress toward it has been slow, and can only be achieved through direct infection with the virus, meaning many will die. A Stanford University study in Santa Clara County9 suggests only 2.5 percent to 4.2 percent of the population have had the virus. Another COVID hotspot in Gangelt, Germany, suggests 15 percent10—higher, but still nowhere near the 70 percent necessary for herd immunity. Given the dangers inherent in waiting on herd immunity, our best hope is a vaccine.
A key concern for effective vaccine development is viral mutation. This is because vaccines train the immune system to recognize specific shapes on the surface of the virus—a composite structure called the antigen. Mutations threaten vaccine development because they can change the shape of the relevant antigen, effectively allowing the pathogen to evade immune surveillance. But, so far, SARS-CoV-2 has been mutating slowly, with only one mutation found in the section most accessible to the immune system, the spike protein. What this suggests is that the viral genome may be sufficiently stable for vaccine development.
What we know, though, is that Ebola was extinguished due to cooperation between public health officials and community leaders. SARS-CoV ended when all cases were identified and quarantined. The Spanish Flu in 1918 vanished after two long, deadly seasons.
The final outcome of COVID-19 is still unclear. It will ultimately be decided by our patience and the financial bottom line. With 26 million unemployed and protests erupting around the country, it seems there are many who would prefer to risk life and limb rather than face financial insolvency. Applying smart containment principles in the aftermath of the shutdown might be the best way to get the economy moving again, while maintaining the safety of those at greatest risk. Going forward, vigilance and preparedness will be the watchwords of the day, and the most efficient way to prevent social and economic ruin.
Anastasia Bendebury and Michael Shilo DeLay did their PhDs at Columbia University. Together they created Demystifying Science, a science literacy organization devoted to providing clear, mechanistic explanations for natural phenomena. Find them on Twitter @DemystifySci.
Lead image: Castleski / Shutterstock
References
1. Genomic epidemiology of novel coronavirus – Global subsampling. Nextstrain www.nextstrain.org.
2. Covid-19 TrialsTracker. TrialsTracker www.trialstracker.net.
3. Struck, M. Vaccine R&D success rates and development times. Nature Biotechnology 14, 591-593 (1996).
4. Breman, J. & Johnson, K. Ebola then and now. The New England Journal of Medicine 371 1663-1666 (2014).
5. Baseler, L., Chertow, D.S., Johnson, K.M., Feldmann, H., & Morens, D.M. THe pathogenesis of Ebola virus disease. The Annual Review of Pathology 12, 387-418 (2017).
6. Eichenbaum, M., Rebell, S., & Trabandt, M. The macroeconomics of epidemics. The National Bureau of Economic Research Working Paper: 26882 (2020).
7. Kissler, S., Tedijanto, C., Goldstein, E., Grad, Y., & Lipsitch, M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science eabb5793 (2020).
8. D’ Souza, G. & Dowdy, D. What is herd immunity and how can we achieve it with COVID-19? Johns Hopkins COVID-19 School of Public Health Insights www.jhsph.edu (2020).
9. Digitale, E. Test for antibodies against novel coronavirus developed at Stanford Medicine. Stanford Medicine News Center Med.Stanford.edu (2020).
10. Winkler, M. Blood tests show 14%of people are now immune to COVID-19 in one town in Germany. MIT Technology Review (2020).






























