This story was originally published by Knowable Magazine.
You don’t often see the word beautiful in scientific articles. Yet it’s easy to see why cell biologists Niccolò Banterle and Pierre Gönczy used the word when describing a crucial cell structure called the centriole in a recent review. The scientists, of the Swiss Federal Institute of Technology Lausanne, are helping to provide a more precise view of how our cells construct this microscopic marvel.
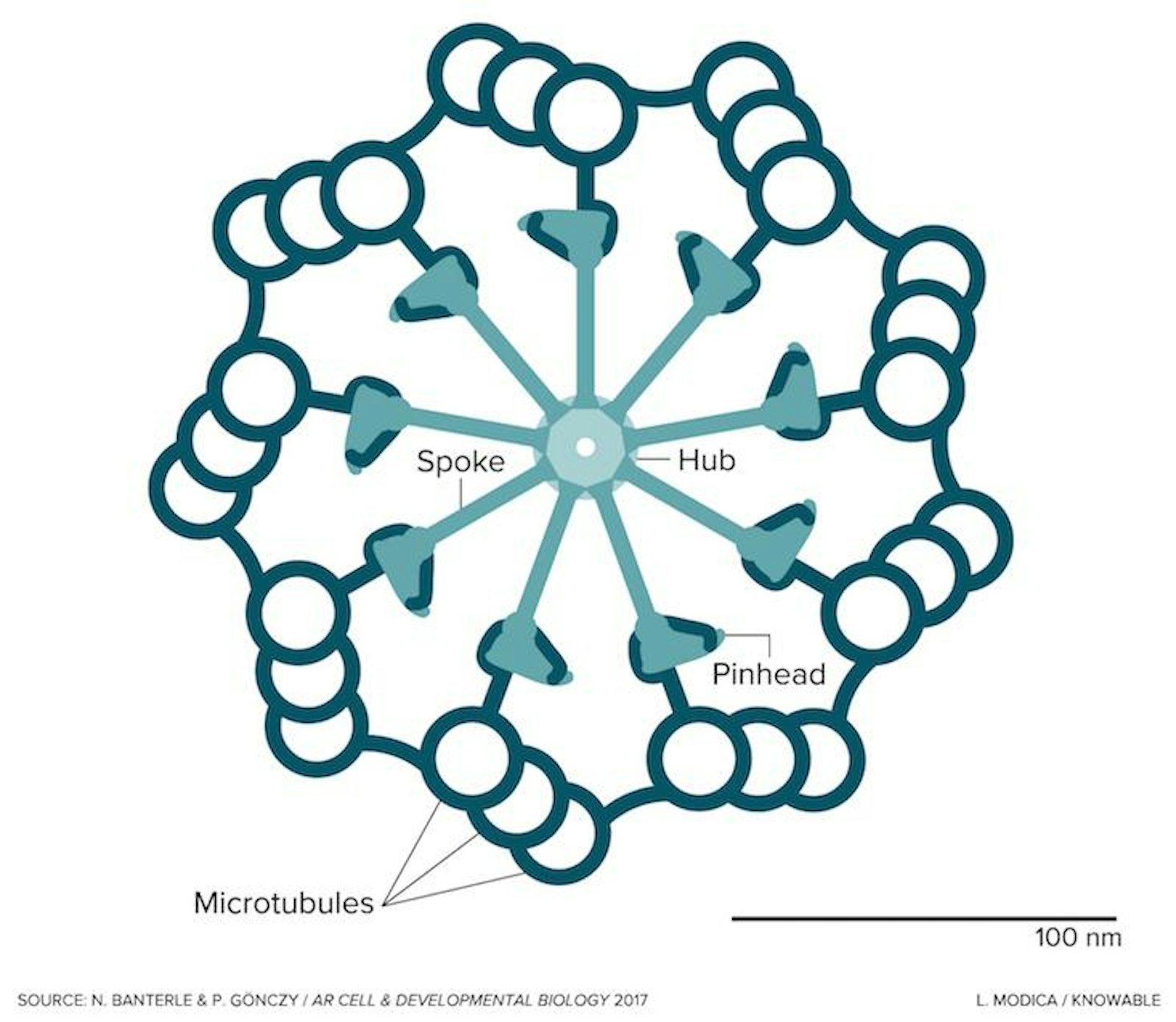
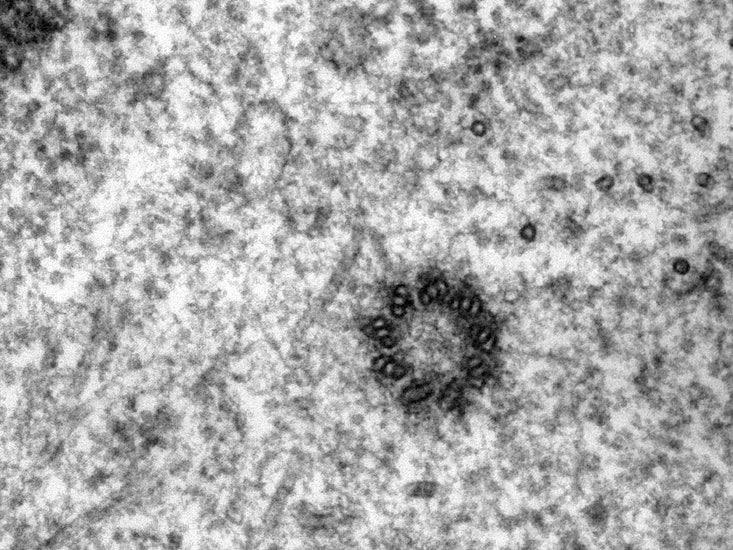
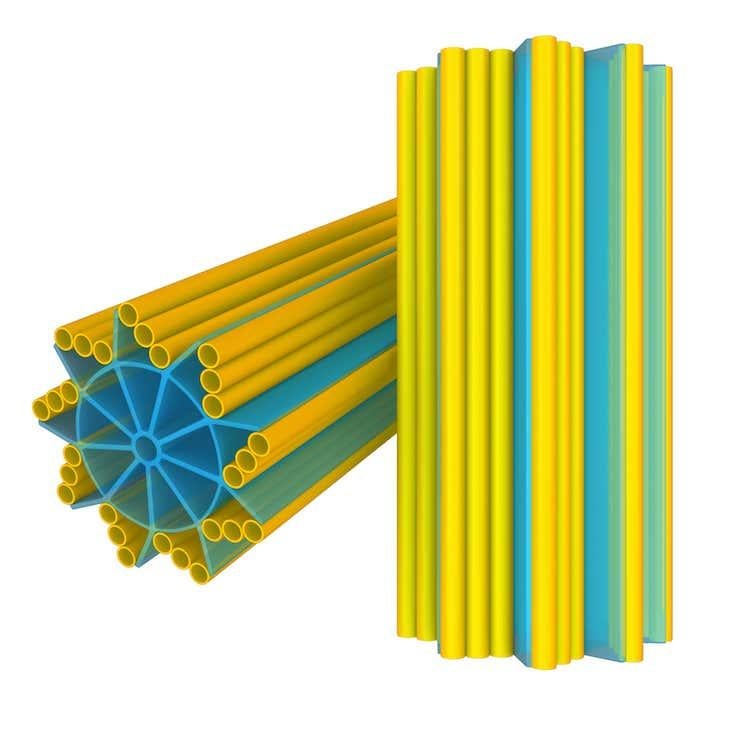
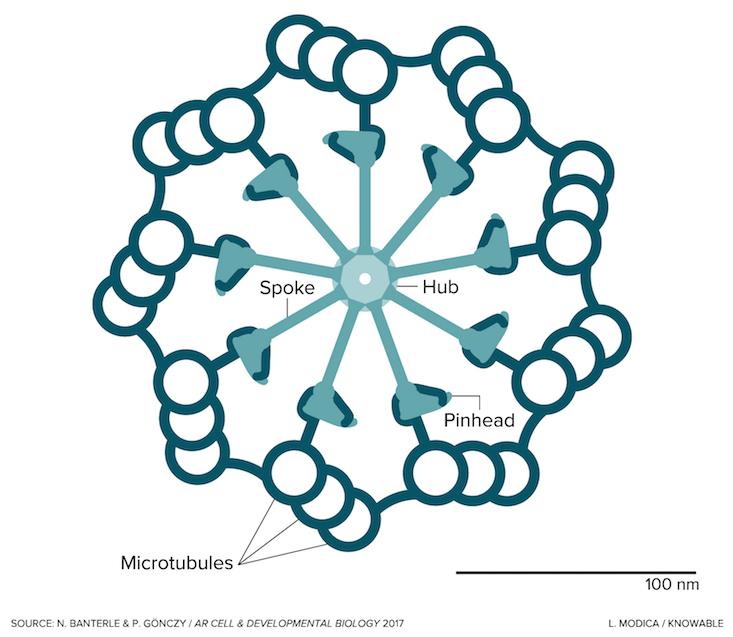
Seen in the image in cross section, the centriole is a cylindrical cell organelle built of nine elegant groupings of three hollow tubes known as microtubules. Each centriole is about one-300th the width of a thickish human hair (about 250 nanometers) in diameter and as much as twice as long. It sits in an area of the cell that looks distinctly fuzzy under the microscope; that fuzzy spot plus the centriole are together known as the centrosome. Each human body contains trillions of them. Centrosome disorders have been implicated in a variety of birth defects, such as certain forms of dwarfism and microcephaly, and may have a role in cancer.
Centrioles have existed for over two billion years and are found in a wide variety of life forms that may use them in different ways. In single-celled organisms such as Euglena or Trypanosoma brucei (the parasite that causes sleeping sickness), centrioles form the core of a flagellum, a whipping appendage that enables cells (including human sperm cells) to propel themselves. That’s probably the job that centrioles first evolved to do, says Gönczy.
Over time, though, the same structure was adapted for another function: fine-tuning the orderly division of cells. Every one of us starts out as a single fertilized egg cell, which then divides into two daughter cells that each produce two daughters of their own, and so on. Getting enough divisions done in the right way and in adequate time is crucial for correctly structuring developing tissues.
Here’s what goes on as cells divide: At the start of division, the centrosome splits in half, designating two centrioles to each portion. The two resulting centrosomes move to opposite sides of the cell. Microtubules sprouting from each centrosome then grow toward the cell’s center, where the chromosomes containing the cell’s genetic material are lined up, waiting. Microtubules latch onto each chromosome, and then—at the right time—drag it toward the pole they are attached to. This way, the cell makes sure that each daughter gets a copy of every bit of DNA.
Centrosomes aren’t strictly required for this, explains cell biologist Anna Akhmanova of Utrecht University in the Netherlands. “Experiments show that many cells can also divide without them, but the entire process then becomes very messy,” she says. For one thing, it takes the cell much longer to properly organize the microtubules for division. For another, chromosomes might end up in the wrong cell or be lost altogether. The process still sort of works, but the centrosome makes it robust, Akhmanova says.
Of course, the result of division is that a cell now has only two centrioles. To coordinate the next division, it will need four, so it makes two more before it divides again. Here’s how that happens: A new centriole is born on the side of an old one in a distinct position marked by the accumulation of an unusual, somewhat suicidal protein called PLK4. “Most of the time,” Gönczy explains, “newly formed PLK4 proteins mark themselves for destruction and are recycled by the cell.” But once in a cell’s life, they pile up in one spot on the side of each old centriole. And a brand new centriole is built.
To guide the construction of that new centriole, the cell first builds a scaffold around the old centriole. That scaffold captures nine pairs of a protein called SAS-6, which is the originator of the centriole’s striking ninefold symmetry. The SAS-6 proteins form a circular hub. On that hub, nine spokes—part SAS-6, part other proteins—then attach, translating the symmetry of the SAS-6 hub into the developing structure. At the “pinheads” at the end of the spokes, first one, then two, then three microtubules form and then merge together into a sturdy, full-grown centriole.
That sturdiness is important, explains Gönczy, because the centrioles undergo a lot of wear and tear. Last year, a group of scientists led by biologist Tim Stearns of Stanford University studied mutant human cells that had centrioles with single, instead of triplet, microtubules. They observed that during cell division, many centrioles were torn apart.
Human cells can’t survive without centrioles or centrosomes, but some of us do manage to get by with fairly faulty ones, explains cell biologist Fanni Gergely of the University of Cambridge in the UK. “Some people have mutations in the genes coding for centrosome proteins that lead to the production of rather unusual centrosomes,” she says. Others produce cells containing too many.
The results of those errors are usually similar: Cell division occurs, but with a large delay that’s made worse by biological controls that eliminate irregularly or slowly dividing cells. “To a developing embryo, which has only a limited amount of time to grow all the cells it needs, this is a big problem—especially in areas such as the brain where the number of cells is largely fixed at birth,” says Gergely. Low cell numbers mean that people with some of these mutations may be born with very small bodies or, more commonly, just small heads and brains—a condition known as primary microcephaly that is linked to cognitive impairments.
Gergely is trying to understand how that happens. She’s intrigued, too, by what similar mutations do if they emerge later in life. “The same centriolar defects that delay cell division in embryos may cause cancer in adults,” she says. A recent screening study, for example, revealed that many cancer cells have extra centrioles. The centrioles also are often abnormally long, causing them to produce too many microtubules, which can lead to an inaccurate partition of the chromosomes and make cancer cells more invasive. Gergely says that some new, experimental therapies aim to sabotage cancer-cell centrioles, though the results of these trials are still to come.
Centrioles are not just important during cell division: Microtubules also grow from the centrosomes of cells that aren’t dividing. Instead of dragging chromosomes around, they form part of the cell’s inner skeleton—a mesh of protein filaments that provides strength and support, enables a cell to move and coordinates the transport of materials within it. Scientists are learning how the centrosomes help these microtubules grow.
At high concentrations in the lab, the proteins that microtubules are made from—called tubulin—join together into hollow tubes entirely spontaneously. In cells, they never do, Akhmanova and Utrecht University colleague Jingchao Wu wrote in 2017 in the Annual Review of Cell and Developmental Biology.
“The cell doesn’t want to grow microtubules everywhere,” Akhmanova says. So it keeps things in check, again by building a scaffold to serve as a guide inside that microscopically fuzzy area surrounding the centriole. The tubulin proteins will click together, “like Lego blocks,” she says, only with this scaffold’s help.
The centrosome is not the only place where this happens, Akhmanova adds. As cells specialize, microtubules may sprout at other places. In this way, every cell type grows the skeleton it needs to move around or to transport, absorb or release molecules, without requiring detailed, individual instructions for each item. “Cells build themselves, without any ‘plan’ of where to put what,” Akhmanova says. Yet it all works beautifully.
Tim Vernimmen is a freelance journalist based near Antwerp, Belgium. He writes about the science of life.
Editor’s note: This story was updated June 4, 2018, to clarify the affiliations of cell biologists Pierre Gönczy and Niccolò Banterle. They work at the Swiss Federal Institute of Technology Lausanne; the Swiss Institute for Experimental Cancer Research, cited in an earlier version of the story, is a department within EPFL.
Knowable Magazine is an independent journalistic endeavor from Annual Reviews.