The announcement of synthetic diamonds in 1955 was met with the same kind of alarm and skepticism that greeted claims to have made alchemical gold in the Middle Ages. Could these “fake” gems, created by a team at the General Electric research laboratories in Schenectady, New York really match the genuine article? One anonymous critic from California captured a widespread suspicion in blunt terms when he wrote to GE, saying:
You can’t make real diamonds for they are nature grown. You can’t make gold; no one can. They dig gold out of the ground and also diamonds. But no one can make them with a machine. That is just a lot of bull.
Yet what if it were true that diamonds really can be manufactured? When GE revealed the discovery, the stock of the De Beers diamond cartel in South Africa, which dominated the global market, plummeted. It seemed like a rare and precious commodity was about to be supplanted by an artificial form that could be fabricated by the ton, mirroring a millennia-old concern about the devastating power of fakes. Concerns over the devaluation of gold currency led the Roman emperor Diocletian to ban alchemy in the third century, and worries about counterfeiting and debased coinage also lay behind the condemnations of the art by Pope John XXII in 1317 and of King Henry IV of England in 1403.
This, though, was no alchemy: The GE diamonds were perfect chemical replicas of the real thing. Was it the end of a billion-dollar market?

The answer was no. “Fake” diamonds are cheaper, and for industrial uses they have utterly eclipsed their natural counterparts. But at the luxury end of the market—gemstones for jewelry—artificial diamonds account for only 2 percent of global sales. How come?
When it comes to luxury and exotic materials, the competition between fake and real is partly a technical, chemical affair: how to create a good imitation, and how to spot it. But, as artificial gold and diamonds show, there is a deeper level to it, which is about something very human and socially constructed: the concept and value of authenticity.
There are few better examples of the social implications of chemical “fakery” than the synthetic dyes of the 19th century. Medieval and Renaissance cultures were virtually color-coded hierarchies. Crimson and scarlet garments were for cardinals, bishops, popes, and monarchs, echoing the ruby-purple of the emperor’s robes in classical Rome. Clothing displaying other rich colors was a mark of wealth; black in particular came to signify the conspicuous consumption of the affluent merchants, who could afford cloth dyed in several expensive dyes until it took on this somber shade. Cheap substitutes would fool no one—they faded fast in sunlight or washed quickly from the threads.
Purple, with its associations of antique nobility, was especially resonant. But how challenging it was to make! When the wife of Napoleon III, the glamorous Empress Eugénie, visited England in 1858 dressed in a crinoline made from swathes of glorious mauve-hued cloth, the English went crazy to imitate this display of continental haute couture. Even Queen Victoria rather dowdily followed suit. The timing could not have been better for the young chemist William Perkin, who discovered in 1856 how to make a strong and permanent purple dye from an extract of the abundant coal tar, the residue from the extraction of gas from coal. Tempted at first to call it Tyrian purple to capitalize on the exclusive associations of antiquity, Perkin soon realized that nowadays French fashion counted for more, and it became “mauve,” after the French word for mallow, a plant with purple flowers.

This too seemed a kind of alchemy, as Charles Dickens’ journal All The Year Round acknowledged in 1859:
Alchemists of old spent their days and nights searching for gold, and never found the magic Proteus, though they chased him through all gases and all metals. If they had, indeed, we doubt much if the discovery had been as useful as this of Perkins’s [sic] purple.
But the disdain expressed toward the garish colors that Perkin’s purple, and related dyes such as magenta, made accessible to the general public from the 1860s had within it a clear distaste for the arriviste. Now that simply anyone can wear what once marked you out as a person of consequence meant that new ways were needed to arbitrate social distinction. “Never trust a woman who wears mauve, whatever her age may be,” wrote Oscar Wilde in The Picture of Dorian Gray. “It always means they have a history.”
With chemistry in full bloom in the later 19th century, all the material signifiers of class and culture were under threat. Expensive ivory, allegedly in short supply in the mid-century (although the rumor was false), could be faked with nitrocellulose, a plastic discovered in 1845 by the Swiss-German chemist Christian Schönbein. The British inventor and metallurgist Alexander Parkes turned this sticky mass into a hard substance that he patented as Parkesine in 1862, selling it as an ivory substitute for combs, buttons, and cutlery handles. Parkes’ product was poor—it cracked and caught fire—and his business folded in just a few years. But at much the same time, the American brothers John and Isaiah Hyatt found that nitrocellulose could be made malleable and workable by adding castor oil or camphor, which they marketed with more success as celluloid.

It wasn’t the perfect substitute by any means, not least because it is so flammable: nitrocellulose was also used as a fluffy explosive called “gun cotton.” There are tales of celluloid billiard balls, masquerading as ivory, producing a sharp detonation on contact: not enough to do anyone harm, although John Hyatt attested that the loud cracks might lead to guns being drawn in crowded billiard bar-rooms. The use of unstable nitrocellulose as an enamel substitute in dentures was an even riskier venture.
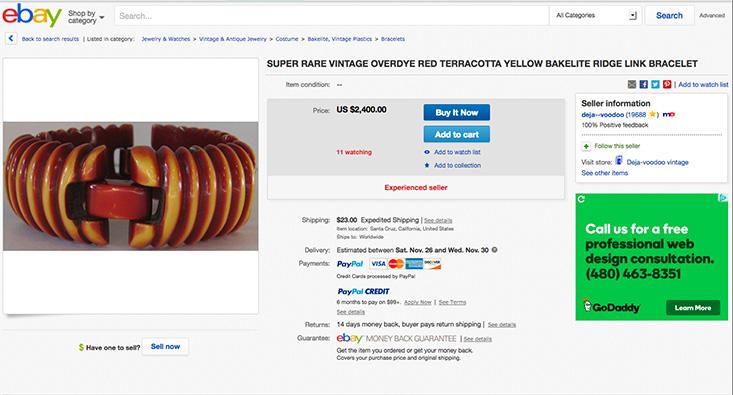
Celluloid was semi-natural, a plastic made from plant cellulose. The era of truly synthetic plastic began with the invention of Bakelite by Leo Baekeland around 1907. This dark resin was a polymer made from phenol (another coal-tar extract) and formaldehyde. It could be easily molded, but once hardened it retained its shape even when heated. This property, and its electrically insulating character, made it well suited for making components in the growing electronics industry. But the dark sheen resembled amber and mahogany, and so it was soon to be found imitating these expensive materials in jewelry, handles, and household devices such as radio sets. Still today buyers on eBay fret over how to distinguish real amber from Bakelite imitations.
Fakes can now be nearly as complex as the objects they copy.
The initial promise of plastics, then, was to provide ‘luxury for all’: materials resembling those only the wealthy had previously been able to afford. They were egalitarian materials: as cultural critic and philosopher Roland Barthes put it, “their use is historically bourgeois in origin … they aimed at reproducing cheaply the rarest substances, diamonds, silk, feathers, furs, silver, all the luxurious brilliance of the world.”
But if individuals of low status can pass themselves off as high-status—richly garbed, wearing faked gold and jewels—what then can survive of the social order? We might have moved beyond the hierarchy of princes, lords, and bishops, but not beyond the need for a pecking order and a narrative to back it up. It’s to that narrative that we returned in the age of the fake, both to create new emblems of status and to defend old ones.
Mixed up with the human code of privilege and power is an ancient belief in the moral authority of nature’s divine handywork. “Making” is always a compromised, ambiguous affair; beyond even the rhyming coincidence with faking, we have a word in English that can connote either activity: forging. It seemed entirely consistent with the superiority of God’s creations when the 17th-century pioneer of microscopy Robert Hooke found natural objects (such as insects) to be far more intricately and delicately formed at the tiniest scales than the crude artifacts of humans.
Modern science has shifted this balance. Fakes can now be nearly as complex as the objects they copy (or in the case of fake diamonds, essentially identical). One response to this intrusion is to move the boundary deeper into nature, looking for subtler marks of authenticity: Real and fake diamonds tend to have different spectroscopic signatures of light absorption, while X-ray crystallography, chromatography, and mass spectrometry are the high-tech methods used for detective work on pigments to authenticate artworks.
Another response to the increasing perfection of the fake, though, is to change the narratives behind authenticity and desirability—to assert them on different terms. Sometimes the mere passage of time is enough to elevate the fake itself to an object of desire. The “retro” value of genuine Bakelite products, for example, now makes them sought-after, and liable to be forged from more recent, cheaper plastics. The fake, invested with its own unique history, has become the real deal, and fetches a premium price to match. Items of “vintage bakelite” jewelry are listed on the online auction site eBay for thousands of dollars, despite the fact that they are essentially plastic trinkets.
Alternatively, the fake might itself stake a claim to moral authority, as in the case of fake fur, or of fake rhino horn, created using 3-D printing technology in an effort to ease poaching of the endangered animals.
The diamond industry, aware of the flexible nature of how we assign authenticity, is trying to re-invent the narrative surrounding its product. Concerned that synthetic diamonds are starting to enter the high-end jewelry market (sometimes surreptitiously), a cartel of natural diamond mining companies, including De Beers, called the Diamond Producers Association has launched a campaign to promote its products. The rhetoric—“Real is Rare”—speaks volumes about today’s distinctions between “real” and “fake”: If you really love someone, the campaign videos insist, you’ll settle for giving them nothing but a “real” gem. A fake gem becomes a sign of a faked affection. That the artificial gems are probably at this point still more rare than natural ones, and are as “real” a diamond as any other in chemical terms, is beside the point here. The “genuine article” is being protected by a narrative of natural authenticity that—ironically, given the pollution and questionable human-rights records of past diamond mining—draws from that associated with green movements.
Manufacturing such large diamonds is not easy in any case. As well as GE’s high-pressure, high-temperature (HPHT) method that squeezes carbon to diamond, it’s now possible to grow diamond by letting carbon atoms settle from a vapor onto a growing diamond surface, a technique called chemical vapor deposition (CVD). Initially HPHT synthesis could produce only very small diamonds—fine for making abrasive grit, not so much for necklaces. And CVD was best suited to making diamond coatings, which again was fine for cutting tools but not jewelry. Today it’s feasible to grow larger, gem-quality diamonds artificially, but at a price scarcely much lower than that of natural diamonds. And it’s not easy to make these artificially gems perfectly colorless—impurities can give them a yellow, brown, or gray tint. Both for these reasons and our apparent continued belief in the value of a “real” diamond, natural diamond retains almost complete control over the gem market.
The superiority of the “nature grown” substance is of course a strong selling point for health foods and “natural” cosmetic and pharmaceutical products. That isn’t to suggest such claims are totally fictitious: Some of the artificial food colorings that stemmed from William Perkin’s coal-tar dyes really were toxic. But the narrative often insinuates an almost moral authority of the “real” over the “fake.”
Witness the arguments over natural versus synthetic vitamins. “Many vitamin and mineral supplements are manufactured synthetically with chemicals and do not come straight from their natural sources,” one web site, www.GlobalHealingCenter.com, warns:
They are made to mimic the way natural vitamins act in our bodies. Natural vitamins are derived directly from plant material containing the vitamin, not produced in a test tube … Evolution has dictated that we eat the food we can gather from the earth, not the food we create in a lab.
Other sites determined to defend the superiority of natural provenance warn that, even if the molecules are identical, they are synthesized by a different pathway, using different reagents, than those in nature.
It’s true that some claim the natural vitamins—but not the synthetic variants—come with a tranche of enzymes, ions, minerals, and other ingredients that control the way the body recognizes, metabolizes, and uses them. Others say that a synthetic variant might contain impurities that the natural compounds do not. But if these things are truly important for the physiological effects, they can be addressed in principle. The rhetoric, however, sets out to persuade us that a synthetic substance can never be equivalent to a “natural” one, however much they might look identical. And it’s a story that works: Whole-food supplements, which surely qualify as luxury items too, have been one of the most quickly growing segments in the industry.
This distrust of the “fake” and reverence for the “real” and “authentic” seems to be deeply ingrained in human nature. We will find a rationalization of it, one way or another, and construct narratives to keep the distinction alive. For in the end that distinction seems to look not outward to the material world but inward to the self. Philosophers from Jean-Jacques Rousseau to Jean-Paul Sartre have interpreted the idea of authenticity in personal, individualistic terms as a freely chosen response to circumstances, something like Polonius’ dictum in Hamlet: “To thine own self be true.” Psychological studies suggest that sense of personal authenticity inflects how we function in the world: our sense of autonomy and self-esteem, what goals we pursue, how we relate to others. We reject the fake smile, the insincere action; we disdain artifice and pretense. It’s entirely to be expected, then, that the policing of fakery is socially constructed. What matters is not so much how the object is constituted, what chemistry created it, what imperfections it has. What matters is what it says about us.
Philip Ball is a writer based in London. His latest book is The Water Kingdom: A Secret History of China.






























