For years, Ali Khodadoust walked around with his heart literally open to the world. In 2012 surgeons replaced his aortic arch and unknowingly planted bacteria. The bacteria secreted a sticky biofilm and burrowed a channel through his chest, creating a peephole to the open air.
It was a dangerous intimacy. The antibiotics the elderly man dutifully swallowed every morning to fight the infection didn’t kill the bacteria. So doctors inserted plastic tubing into his shoulder, funneling antibiotics directly into his bloodstream. But antibiotic after antibiotic failed. After three years, Khodadoust an ophthalmologist in New Haven, Connecticut, was referred to Yale-New Haven Hospital for treatment. Tawny pus oozed out of an opening the size of a pencil eraser on his chest. Strands of bright blood streaked the pus. At any moment, the bacteria could move into his blood, triggering septic shock and killing him.
To destroy the nasty bug, surgeons would need to slice out the infected tissue, wash out his heart cavity, and replace his aortic arch, again. But they were wary of performing heart surgery on older patients, especially given that the operation would require cutting through a bacterial sheath. They decided that surgery was too risky and postponed operating. Another group in Texas rejected him. Finally, Khodadoust’s last hope, a hospital in Zurich, turned him away.
While Khodadoust was struggling to survive, a microbiologist named Benjamin Chan was working away at an experimental evolution laboratory just one mile to the north. Chan was investigating bacteriophages in the lab of Paul Turner, a professor of ecology and evolutionary biology at Yale. From the Greek phagein, to devour, bacteriophages, or phages, are the viruses that devour bacteria. Phages thrive where bacteria do—which means pretty much everywhere. No organisms on Earth are as ubiquitous or diverse as phages. We touch phages every time we play in the ocean, nibble our kale salads, or kiss. Billions of years of evolution have made phages the ultimate bacterial killers—quiet, stealthy, and effective. But, curiously, no hospitals in the United States currently treat patients with phages.
Khodadoust didn’t know this at the time, but Chan was about to make an exception out of him.
I sat across from Chan one afternoon in his office in Osborn Memorial Laboratories, a cathedral-like building on Yale’s Science Hill (disclaimer: He is a friend of mine). Sunlight streamed through large windows. A hot plate—for food, not science—was wedged between microbiology textbooks on his bookshelf. Perched at his desk in a trim vest, a skinny tie, and lavender argyle socks peeking out of his Oxfords, Chan looked more like a member of an eager indie band than an obsessive microbiologist.
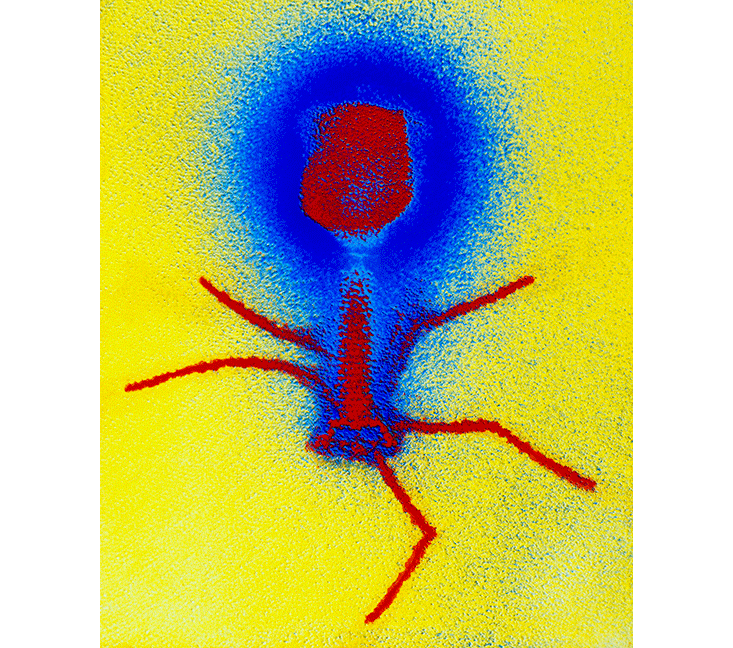
My face must have crumpled when Chan said he planned to infect a fragile 80-year-old with an experimental virus. He reassured me: “Phages infect only bacteria.” In fact, phages often target just a single bacterial species, or only a few strains of a single species. A phage is an intricately shaped key that fits but a single keyhole, a receptor on the bacteria’s cell wall. Once a phage unlocks its target, it slips its genome inside and turns the bacterium into a furious phage-copying machine. Eventually, the bacterium bursts, releasing hundreds of phage clones. Only a bacterial carcass is left behind. (Some phages take a quieter route, inserting their genetic code into a bacterium’s DNA so that each time the bacterium reproduces, the phage is copied too.)
This stands in distinction to how antibiotics operate. Where antibiotics are indiscriminating gourmands, destroying any bacteria in their path, including much of the bacterial community keeping us healthy, phages are refined, restrained gourmets. Phages slip through sticky biofilms, infect their targets, and tidily eliminate offending bacteria and themselves, leaving a patient’s microbiome untouched.
Their unique method of action could allow phages to become powerful weapons in the war against superbugs—bacteria with names like MRSA, C. diff, and CRE that, like Khodadoust’s, can evade most antibiotics. Superbugs lurk in the shrink-wrapped chicken at the grocery store, on train seat cushions, and on freshly made hospital beds and sicken 2 million Americans each year, killing more than 20,000. If antibiotics become powerless and superbugs take over, millions of routine procedures—organ transplants, cancer chemotherapy, even basic dental work—become potentially dangerous. Phages could open a second front in the war. Except, for a variety of reasons, they haven’t—not yet.
Phage therapy began in the early 20th century with an unpaid volunteer at the Institut Pasteur in Paris collecting feces. Microbiologist Felix d’Herelle was tasked with investigating a dysentery outbreak among French soldiers during World War I. Curious why some soldiers became deathly ill and others only mildly sick, d’Herelle grew the soldiers’ fecal bacteria in his laboratory. Some of the bacteria lawns, he noticed, were speckled. Something invisible was killing the dysentery bacteria in tiny spots. In the bacteria from recovering soldiers, the spots—what we now call plaques—seemed to multiply. He surmised that these invisible “microbes of immunity” could be helping the patients recover.

Eager to test out his theory, d’Herelle harvested these microbes—what he and his wife named phage—and administered them to a 12-year-old sick with dysentery. The patient recovered quickly. Encouraged, d’Herelle set up shop in Paris, calling it the Laboratoire du Bacteriophage. Like a parfumier with scents tailored for different occasions, he mixed and sold bacté-intesti-phage for diarrhea, bacté-staphy-phage for skin infections, and bacté-rhino-phage for colds. Phage fever spread beyond France—d’Herelle traveled to Georgia to help establish the Eliava Institute for phage therapy in 1923 and in the 1940s, Eli Lilly sold a series of phage-based treatments in the U.S.
A phage is an intricately shaped key that fits but a single keyhole.
It was a strong start for a promising therapy. But another scientific breakthrough would soon trump interest in phages. In 1928, Alexander Fleming discovered penicillin in the mold juice he had mistakenly produced in his messy laboratory. Penicillin was a miracle drug: It needed none of the artistry of phage mixing, cured consistently, could be manufactured in vast quantities, and could sit for months during shipping. It also arrived just when it was direly needed: World War II. Phages, in contrast, seemed an invisible, capricious by-product of bacteria that sometimes cured infections, but frequently did not. At the time, before the era of DNA and molecular biology, it was not even clear what exactly, a phage was. By the 1940s, antibiotics were being produced en masse in the U.S. and Europe. Phages were tossed into the dusty cabinet of medical castaways.
But antibiotics weren’t adopted with equal enthusiasm everywhere. In the Soviet Union, they were prohibitively expensive. So, while phage research waned in Western Europe, it raced ahead in a network of Soviet and Georgian laboratories centered at the Eliava Institute. By the 1980s, Georgian labs were pumping out two tons of phage sprays, powders, and patches each week. Most was funneled directly to the Soviet military. But research was published in Russian and Polish, and did not, by and large, make it to the States.
Things were different, though, in western molecular biology laboratories, where phages were vigorously studied. Those studies revealed that genes were made of nucleic acids—not proteins—and uncovered how those genes were controlled. Biologists mined phages for many of the enzymes routinely used in laboratories today. And in 1976, the first genome to be sequenced was a phage genome. The powerful and controversial CRISPR-Cas gene editing technology derives from a technique bacteria use as a defense against phages. Eventually, a phase renaissance began in the U.S., sparked in part by scientific exchanges between U.S. and Russian academia.
Chan’s own phage hunting began at OmniLytics, one of a handful of American companies selling phages to farmers. At OmniLytics, Chan searched for phages to protect livestock from Escherichia coli O157:H7 and tomato crops from plant epidemics. We already eat phage additives constantly, Chan told me. Hotdogs and sandwich meats are routinely sprayed with ListexTM, a cocktail of several phages approved by the FDA in 2006 that protects against Listeria monocytogenes. A competing phage company, Intralytix, offers SalmoFreshTM spray that targets salmonella contaminating poultry, fruits, and veggies. Unlike food additives, phages don’t need to be listed on nutrition labels, making it hard to tell if you’re snacking on virus spray. And, conveniently, phage sprays are organic. Omnilytics advertises phages as a crunchy, all natural alternative to pesticides: “Nature provides a better cure.”
When Chan moved to New Haven in 2013, he wanted to investigate phages as human medicines. He promptly emailed the then-president of the Yale-New Haven Hospital on arrival to say he was a phage hunter looking for a patient to help. In short order, Chan met Khodadoust’s doctor, Deepak Narayan, picked up his prize (a small tube of pus on a throne of ice), and acquainted himself with his bacterial target. He planted the pus sample in a mixture of broth and agar. Khodadoust’s bacteria, Pseudomonas aeruginosa, grew furiously and fragrantly—“it smells kinda nice, sweet, like artificial grapes,” Chan told me. He stocked a freezer with small tubes of the bacteria and tested antibiotic after antibiotic against it. The bacteria proved its hardiness. Then, Chan began his quest for a phage that would kill it.
Phages seemed an invisible, capricious by-product that sometimes cured infections, but frequently did not.
The diversity of phages dwarfs the diversity of all other life on Earth. Everywhere scientists look—in soils, in caves, in the deep ocean—they find millions of new phages. The trouble is, phages are less than 100 nanometers long and there are about 1032 of them out there. If a phage were the size of a grain of sand, you could fill 1,000 Earths with phages. No map exists to help a phage hunter track down viral treasure. The phage Chan sought could exist anywhere contaminated with Pseudomonas—in humans, in hospitals, or in nature.
Chan searched high and low—really low. He wasn’t above asking his lab mates and friends to chip in with their own fecal samples. Other biologists in the building heard about Chan’s hunt and chipped in with whatever was on hand. “The fish people upstairs”—ecologists studying freshwater trout—gave Chan leftover samples they had collected from New England waterways. Chan compiled dozens of samples from lakes, puddles, sewage, compost, and soil. Each small vial he collected brimmed with bacteria and phage.
Then, in his lab, Chan made the phage easier to find. He mixed each sample with a few drops of Khodadoust’s Pseudomonas bacteria. Only the matching phage could infect the Pseudomonas cells and multiply. Like a vintner filtering grape skins, seeds, and leaves out of wine, Chan then poured each mixture through a filter 100 times narrower than a human hair. Only the purified nectar—a pool of phage—remained.
Finally, to test if the nectar could truly kill Pseudomonas, rather than just multiplying inside them, he mixed Khodadoust’s Pseudomonas with the purified phage, painting the mixture of bacteria and phages on Petri dishes and incubating the dishes overnight. A bull’s-eye of bacteria death, a plaque, would form if a phage successfully entered and burst Pseudomonas cells, released more phages, and gradually spread outward across the bacterial lawn.

Late one night, months after he began his search, Chan found the golden phages. Standing alone in his gothic, high-ceilinged laboratory, he stared in disbelief at the Petri dish he held in his gloved hands. Clear spheres shone against the dish’s emerald background, like planets of different sizes floating across a dusky sky. He held a bacterial graveyard. After carefully plating hundreds of Pseudomonas dishes, and garnishing each with a meticulously prepared sample, he had found a phage key capable of unlocking Khodadoust’s bacteria, entering, and causing chaos within.
He squinted at the label on the side of the Petri dish. Dodge Pond. The fish people upstairs had delivered. Water from a bucolic Connecticut pond harbored bacteria killers more powerful than the strongest antibiotics, at least when it came to Pseudomonas. Chan quickly confirmed that his killer phage could also slip through protective biofilms that Pseudomonas had created on Khodadoust’s artificial aorta.
Then came the harder test—would the Pseudomonas evolve to resist the phage? No therapy is evolution-proof. One of the major critiques of phage therapy is that it will fail the same way antibiotics have failed: Bacteria can evolve phage resistance. (This is why phages are often administered as cocktails. If bacteria evolve resistance to an individual phage, ideally, other phages will still be potent.) To test this possibility, Chan let Khodadoust’s bacteria evolve in the presence of phages. As expected, after a single night, the bacteria had become immune. But were the phage-resistant bacteria now weak in some other way? Chan spread the newly resistant bacteria across a Petri dish and added ceftazidime—a powerful antibiotic—to the center of the plate. The next morning, a beautiful death halo had formed.
The Pseudomonas had evolved resistance by losing the receptors that Dodge Pond phage entered through. Phage entrance and destruction was now impossible. But without these phage receptors, the bacteria became vulnerable. That’s because receptors for Dodge Pond phage play another crucial role: They pump out antibiotics. When Chan added antibiotics, the drugs—which previously had been ineffective—flowed easily through the bacteria’s cell wall. With no pump to remove the antibiotics, Pseudomonas was poisoned from within. The combination of Dodge Pond phages and ceftazidime had created an evolutionary checkmate.
Even as phages have resurfaced in the U.S., they face a new set of hurdles for use as medicines, not the least of which is FDA approval. “Phages appear to work,” Randall Kincaid, a senior scientific officer at the National Institutes of Health, tells me. “Yet, we don’t have statistical basis in a well-controlled study to state that phages are the reason for recovery. We need to think about this from the perspective of doctors. When a patient walks into a doctor’s office with a bacterial infection, the standard of care is a small-molecule antibiotic. We need to establish unequivocal proof of their benefits for there to be acceptance of phages.”
A handful of early-stage clinical trials have been conducted, with promising results. A Phase I and II trial conducted by AmpliPhi Biosciences Corporation found that a single dose of a phage cocktail worked well against drug-resistant Pseudomonas aeruginosa ear infections. Another Phase I clinical trial found that a phage cocktail for untreatable infections of leg ulcers was safe, but it didn’t significantly speed patient recovery (Phase I trials are designed to test if drugs are safe; Phase II trials, if they are effective).
But not a single Phase III clinical trial—the final stage of confirming a medicine’s efficacy, requiring at least 1,000 patients—has been attempted. Because phages work on such a different principle than antibiotics, it’s not even clear how to evaluate them. Kevin Outterson, a professor of law at Boston University School of Law and the executive director and principal investigator of CARB-X, an “accelerator” that plans to funnel $350 million to new products fighting superbugs, tells me that “for antibiotics, we understand what type of evidence we want to see in animal models and what type of human studies we need. What a phage trial would look like in humans is a much more open question.”
The diversity of phages dwarfs the diversity of all other life on Earth.
Some of the most obvious benefits of phages also pose issues that frustrate regulators and alarm critics. Foremost is the specificity of phages, which makes them precise tools, but also means that clinical trials need to confirm safety and efficacy for specific bacterial species or strains. While broadly-acting antibiotics are often prescribed without a specific diagnosis, phage treatment requires doctors to precisely identify their patient’s bacteria. This would transform doctor’s visits. Dosing, too, is more complicated for phage treatments. Antibiotics move through the body and are metabolized in predictable ways. But phages are not inanimate compounds. If they find their target bacteria, they amplify. While this could mean that sometimes, only a single dose of phage will be needed, it also means that phage activity is difficult to predict. How much phage should patients be given? And how fast will bacteria evolve resistance? Even when phages perform brilliantly in vitro, in the lab, it is hard to say what they will do in vivo, in the complex and difficult-to-navigate environment of the human body.
There is also the ugly question: Who is to pay for the trials? Clinical trials often cost hundreds of millions of dollars. And compared to cancer drugs, Kincaid tells me, “for infectious disease therapies, by and large, there’s not a lot of return on investment.” Right now, it’s tough to make the case for phage to investors who want to be assured of a high likelihood of success. Phages, harvested from sewage and compost, are natural products and can’t be individually patented. Companies can get around this by patenting phage cocktails or by engineering phage. “Precedent plays a big role in this,” says Kincaid, who ran a biotech company for years before his current gig at the NIH. “Investors are wary of the vagaries of things we don’t know.” He thinks the government needs to play a role in pushing (and paying for) phage research.
The largest phage therapy trial to date, backed by €3.8 million from the European Commission, ran into several of these problems. The trial, called PhagoBurn, originally involved recruiting 220 burn victims with infected wounds from 11 different French, Belgian, and Swiss hospitals. The ambition was to test how two different phage cocktails fared against the standard antibiotic, silver sulfadiazine. After fighting to prove that each of the phages in the cocktail were stable, the trial stumbled over the daunting problem of patient recruitment. To be considered eligible, patients had to be infected with either Escherichia coli or Pseudomonas aeruginosa, but not both. But burn victims are often colonized by many pathogens. Only 15 patients were found to participate in the P. aeruginosa study. The E. coli study was dropped entirely. The shrunken trial continues and results are expected next spring, four years after it began.
There are those who are dismissive of phage therapy. Steve Projan, the senior vice president of research and development and the head of the infectious diseases and vaccines unit at the biotech company MedImmune and formerly of Novartis and Wyeth, put it this way in a 2004 article: “The personal, anecdotal testimonials of former patients who ‘benefited’ from phage therapy is both amusing and sad—we do not hear from those patients whose infections were not cured, for obvious reasons.” Projan writes that money spent trying to overcome all of the hurdles to phage therapy could be better spent elsewhere—like developing new “small-molecule therapeutics,” including antibiotics. While he does not see phages as therapies themselves, Projan does think that studying how phages fight bacteria could reveal other small-molecules that could be the next antibiotics. He declined my request for an interview.
Others are more optimistic. A handful of Eastern European countries routinely use phage therapy. The Ludwik Hirszfeld Institute in Warsaw, Poland uses phages as a tool of last resort, for patients failed by all antibiotics. Since 1980, over 1,500 patients with drug-resistant bacterial infections have been treated and the institute reports that “the majority of patients were cured.” In the former Soviet republic of Georgia, phages are used even more widely. Doctors treat about 20 percent of bacterial infections with phages. The Phage Therapy Center in Tbilisi draws patients from around the world who are suffering from untreatable UTIs, acne, cystic fibrosis, and intestinal infections. The center claims that 95 percent of patients are cured. (Many scientists are skeptical—Georgian phages haven’t been tested by the FDA or the European Medicines Agency.)
Then there is Khodadoust and Chan.
Several months after Chan’s success in the lab with Pseudomonas, it was time to try the treatment on Khodadoust. Khodadoust was brought into the CT procedure room at Yale-New Haven Hospital and two medical residents wheeled in a red cart filled with the emergency equipment needed to resuscitate someone near death. A wave of hot panic rolled over Chan, as he described to me later. Before a small audience of surgeons and medical students, a radiologist poured a bottle cap’s worth of salt-water mixture, full of phage and antibiotic, into Khodadoust’s chest cavity. Chan’s eyes fixed on the neon green zigzag marching across Khodadoust’s heart rate monitor. Each ragged peak, a reassurance that pouring virus directly into a man’s heart had not yet killed him. A day later, Khodadoust was discharged from the hospital. There had been no obvious change in condition.
Chan was unsure if he was more disappointed or relieved. “I was nervous about killing the guy. Either [phage treatment] would work—incredible—or it would go poorly—devastatingly poorly,” Chan said.
Instead, for weeks, Chan heard only silence. A month later, Chan heard through Narayan that Khodadoust had hopped on a plane to visit family abroad. Did Khodadoust feel so healthy and refreshed he decided to travel? Or was he traveling to be with his family to die?
Then, six months after the phage procedure and without warning, Khodadoust walked into Narayan’s clinic one morning. His chest had healed completely. A pus-filled crater closed neatly into an even plane. Even the usually reticent Narayan told Chan that his patient looked like “a million bucks.” For the first time in three years, he was off his antibiotics. There had been no side effects.
Narayan can’t say for sure that Khodadoust’s recovery resulted from the phage treatment. In an ideal world, he would have followed Khodadoust closely after the surgery, monitoring the hole in his chest, testing anything that oozed out for Pseudomonas. Still, he is confident the Dodge Pond phage helped his patient. He learned that five weeks after the phage treatment, another group of surgeons saw Khodadoust to remove part of the graft holding his replacement aorta that had been irritating him. At that time, they tested the graft. Khodadoust was already Pseudomonas-free. He stopped taking antibiotics for the first time in three years and his infection has not returned. A virus from a pond just 40 miles from his house apparently gave him a new lease on life—and gave a new meaning to the term “local medicine.”
Chan, Narayan, and Turner celebrated with a dive into furious writing. They hope to plan a clinical trial of the Dodge Pond phage and ceftazidime mixture. Before they can bring phages to more people though, they must first test it in rats. The team is now working with the NIH’s preclinical services to conduct the in vivo animal testing needed to set the stage for a future clinical trial.
In the meantime, Chan is building a phage library. If you live in New Haven (or have recently used a toilet there), you may be an unwitting donor. He visits the New Haven Water Treatment Plant weekly, picks up samples arriving from toilets across the city, filters them for phages, and tests those phages against plates upon plates of potential superbugs. Hunting has proven successful. Chan has isolated phages for Klebsiella pneumoniae and Enterococcus faecalis bacteria, both of which are drug resistant and cause chronic UTI. And on a recent phage hunting trip in Haiti, Chan collected a phage that attacks the cholera bacteria, Vibrio cholerae.
Sometimes, at least, one person’s excrement can make another’s phage cocktail.
Katharine Walter is a graduate student in epidemiology at Yale University. She studies the ecology and evolution of infectious diseases.