The vampire live on, and cannot die by mere passing of the time,” Dracula’s Abraham Van Helsing tells his colleagues. “He can flourish when that he can fatten on the blood of the living. Even more, we have seen amongst us that he can even grow younger …”
The great gothic novel didn’t invent the idea of rejuvenation through blood. It drew on a belief that persisted from the myths of ancient Greece through the early days of Christianity—and, remarkably, to the present day. As Van Helsing lays out his methods to capture and kill Dracula, he tells his companions they have a special power on their side, “a power denied to the vampire kind”—the power of science. In a remarkable irony, certain scientists in the past decade have taken the side of the vampires, offering evidence that blood from the young, when transfused into the old, may strengthen “vital faculties” and ward off elements of aging.
Bram Stoker wrote at a time when science was countering widely held superstitious and religious beliefs. Is the modern-day resurgence of interest into the rejuvenating powers of blood good science working well, or a step backward into a familiar superstition?

Each of our bodies course with a little more than a gallon of blood, from our blushing cheeks to the teal veins that branch across our arms. The earliest humans to see this vital liquid as a life-giving source were the ancient Greeks. Hippocrates, the Greek physician from 400 B.C. and the father of modern medicine, believed our bodies were controlled by four fluids called “humors”—blood, phlegm, and black and yellow bile. The humors were said to influence our personality, emotions, behavior, and age. The ancient Greeks saw the sanguine heat of blood to be so essential for life, that it was considered its very source.
The Roman poet Ovid, in 8 A.D., published the first instance of a revivifying transfusion. In his epic Metamorphoses, the witch Medea slits an old man’s throat and drains all the blood out of him. She fills his “ancient veins with rich elixir” that revives him. The elixir is not blood but root, herbs, seeds, flowers, and the like. But the point remains: The “new blood” grants him the “vigor of bright youth.”
In a remarkable irony, scientists in the past decade have taken the side of the vampires.
The Greeks weren’t far behind Ovid’s account. After gladiator duels, spectators would pour into the stadium in a mad dash to drink the fighters’ gore, desperate to consume the strength and bravery that must have been flowing through their veins. In 77 A.D., Pliny the Elder observed that epileptic spectators were like “wild beasts” upon the arena, rushing “to quaff the warm, breathing, blood from a man himself.” They put their lips onto a gladiator’s wounds, hoping to suck a cure straight from his veins.
By the 15th century people were trying more direct methods. When all attempts to revive Pope Innocent VIII from his stupor had failed, a physician with a concerning lack of qualifications named Abraham Meyre brought three young shepherd boys to the Pope’s bedside, each about 10 years old, selected as sacrifices and bought for one ducat a piece. Legend has it he drained some of their blood into the Pope, and then transfused some of the Pope’s blood into them. Shortly after, all four died.
Nevertheless, the myth of blood rejuvenation persisted. In the late 16th century, the German physician Andreas Libavius wrote that transfusing the blood of a young, healthy person into the vessels of a sick elder could “bring him the fountain of life and drive away all languor.” In the early 20th century, young blood’s role as an antidote for aging informed the philosophy of Alexander Bogdanov, a Russian physician, polymath, and early Bolshevik. He understood transfusing young blood to be an almost metaphysical experience. It was an ambrosia capable of rejuvenating even the most exhausted human body. His philosophy manifested into a fictional 1908 tetralogy laying out an ambitious vision of a utopian communist society on Mars. His Martians lived to be more than twice the age of Earth’s humans, and to achieve this prolonged lifespan, they transfused themselves with young blood, prolonging youth for hundreds of years. He imagined a large-scale concept of the “blood brother,” where all members of society shared their blood communally.
By the spring of 1928, Bogdanov had received 11 transfusions in total, convinced that they increased his cognitive abilities, his eyesight, and even reversed some of his baldness. After trading one liter of his blood for a liter from a 21-year-old boy later that year, Bogdanov died from his twelfth and final transfusion.
In Faust, Goethe writes that “blood is a juice of rarest quality.” But for most of history humans had no idea what that quality actually was. As early as the 17th century, scientists began to understand that blood was composed of distinct components, each of which had a distinct function in the body. But it took the advance of modern medical science to pinpoint those functions and how they could be used for transfusions.
If you spin blood in a centrifuge, it will separate into three layers. The bottom layer is composed of red blood cells, which transport oxygen from the lungs to the body’s organs. The top layer, a translucent pale yellow, is the plasma, which contains clotting factors, proteins—which transport vitamins and minerals throughout the body—and antibodies, proteins that work with the immune system to fight infection. Without plasma, other cells would have no fluid to travel through. In between those two layers are pale yellow platelets and white blood cells, which help the blood to coagulate and provide immune defense, respectively. Any of these elements can be replaced during
a blood transfusion.
Following the previous centuries’ messy attempts at transfusions between dogs, lambs, and eventually humans, the 20th century saw a revolution in the way blood was controlled, stored, and used as a medicine. In 1901, Austrian physician Karl Landsteiner discovered the first three human blood groups, A, B, and C—which later became O—and went on to win a Nobel Prize for his work. A year later, two of his colleagues, Alfred von Decastello and Adriano Sturli, added the fourth blood type, AB. Far beyond the early days of transfusing the blood of friends, family, and whoever was standing by, doctors could now check the type of donated blood, matching it to their patients. These advances lay the foundation for manipulating and even manufacturing human blood for transfusions.
The modern interest in blood renewal kicked off in the 1990s. That’s when Michael and Irina Conboy, a researcher and an associate professor of bioengineering at the University of California Berkeley, respectively, began mulling one of the biggest questions in aging research: Why, when we get older, do the organs and tissues in the body seem to age together?
“Well, the first question to ask is what’s in common to all tissues, and that’s the circulatory system,” says Michael Conboy. Through a series of veins and arteries, the body circulates the blood. Because the circulatory system delivers all of the nutrients to the tissues in our body, doesn’t that make our blood the main culprit? “Our idea was, what if we took a mouse, took out all the old blood and gave it young blood,” he says. “Would that make it young again?”
Spectators put their lips onto a gladiator’s wounds, sucking the life straight from his veins.
When we get older, there is more inflammation in the body, which comes from an increase in inflammation-type signals in the blood, Conboy explains. Another important change with age comes from cellular senescence, when the cells in the body stop dividing. Senescent cells accumulate in our tissues and organs as we age, which can lead to cancer, disease, and other age-related declines. To test whether these negative changes could be reversed, the Conboys turned to mice.
In 2005, working with a team of researchers at Stanford, the Conboys published a study in Nature showing the effects of heterochronic parabiosis—splicing together the circulatory systems of two animals of different ages—which in most cases are mice. The researchers detached the side flanks of skin from a young and old mouse and sutured them together like conjoined twins. The young mice were around 2 to 3 months old, and the older mice were 19 to 26 months. In human years, it was like hooking a 20-year-old up to a 70-year-old. As they healed into each other, their two circulatory systems fused together—the blood from one flowing seamlessly into the veins of the other.
The older mice were were able to bounce back more quickly after injuries, and showed a proliferation of cells in the liver and hippocampus—which is usually the opposite in older mice. The effects of aging seemed to have been reversed—although it wasn’t clear that the effect derived just from blood. The circulatory system of the older mouse also had access to the younger mouse’s organs, which could help regulate the metabolism of the older mouse, help it to heal more quickly, and even supplement it with proper hormones and nutrients. There were other contingencies resulting from being attached by skin. Sometimes, the young mouse would even drag the older mouse around the cage, giving it more exercise.
In November last year, Michael and Irina Conboy published a study in Nature Communications examining the effects of a single exchange of old and young blood between two mice, mixing their blood equally. In this case, the mice were not conjoined as they were in the 2005 study. The Conboys developed a new device that could connect and disconnect the mice at will, which eliminated the effect of sharing organs. The technique was essentially a single mutual transfusion between the mice. That way, the results would show only the effects of the blood exchange, rather than the shared circulation system.
Again, the older mice saw immediate improvements to muscle regeneration and liver health—the fatty deposits nearly disappeared, mirroring the organ of a young mouse—but the Conboys discovered in later research that the old blood was far more inhibitive than the young blood was beneficial. Tellingly, when they mixed together equal parts of old and young blood, the result did not look like middle-aged blood. “It just looks like old blood,” Conboy says. Old blood severely obstructed the young mice’s formation of brain cells, led to an increase of fatty deposits in the liver, and even led to poorer performances during strength tests, where they were hung upside down from a mesh wire. “Again the old animals saw a dramatic improvement, but what was even more interesting to us was the deficits in the health of the young animal from the old blood,” Conboy says. “After those results, we weren’t all that interested in young blood as a medicine. We need to figure out what in old blood is so detrimental.”
Conboy thinks one culprit might be TGF beta, a family of proteins that helps control many of the body’s cellular functions, including cell growth, differentiation, and a signal to shut off a cell’s growth. As we get older, the amount of TGF-beta type proteins in the body increases, releasing a pro-inflammatory signal to the cells that impedes healing and blocks the proliferation of stem cells. If we could instead give cells the proper growth stimulus and override negative factors such as TGF beta, it might be possible to keep the cells growing, restoring the old body’s tissues.
“That’s what we see in the muscle of old mice after the transfusion; it’s able to regenerate just as well as young mice,” Conboy says. “If we could intervene and give more youthful signals to the cells, it would be more than adequate to rejuvenate the tissue.” The real impact of these experiments, then, may be a new understanding of why old blood is so deleterious, rather than reason to inject young blood.
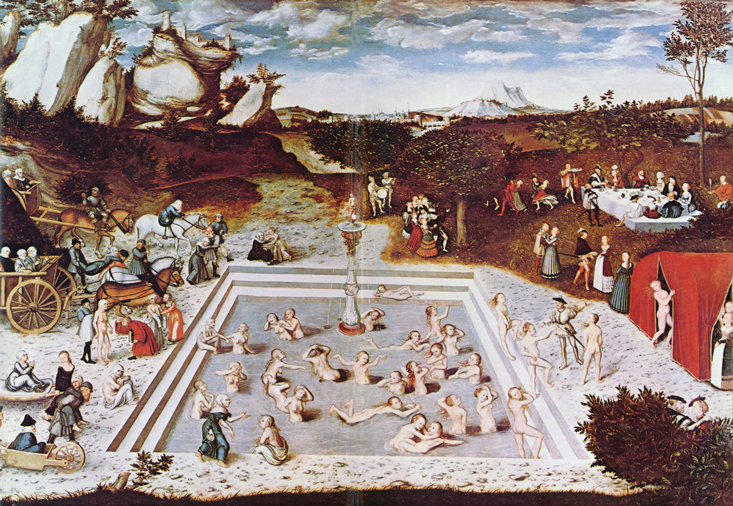
In Lucas Cranach the Elder’s 16th-century painting The Fountain of Youth, haggard women clamber into the flowing waters of a glaucous pool. They frolic, they become softer, more beautiful, and emerge from the other end of the pool revivified, the bodies of their youth restored.
That might be the very vision of a new cohort of medical entrepreneurs promoting the virtues of young blood. One of them is Stanford neuroscientist Tony Wyss-Coray, who has worked to isolate elements in youthful blood that he says can revive aging bodies. He’s started a company called Alkahest dedicated to reversing some of the degradations of Alzheimer’s disease by injecting patients with small doses of plasma from young donors. “Now, I don’t think we will live forever, but maybe we discovered that the Fountain of Youth is actually within us, and it has just dried out,” Wyss-Coray said in a TED talk. “If we can turn it back on a little bit, maybe we can find the factors that are mediating these effects, we can produce these factors synthetically, and we can treat diseases of aging.”
Nobody has capitalized on the parabiosis studies more than Jesse Karmazin, a Stanford-trained physician in California, at least judging from the amount of media coverage he has courted and received. Karmazin has launched his own startup called Ambrosia—after the immortal food of the gods—that is running a trial to test the effects of blood infusions from the young into the old. (Following a rumor that PayPal cofounder and venture capitalist Peter Thiel was interested in Karmazin’s trials, the TV show Silicon Valley spoofed the process, featuring obnoxious tech giant Gavin Belson getting transfusions from his “blood boy.”)
Ambrosia’s ongoing trial admits people over the age of 35—of more than 100 patients, the youngest patient is 39, and the oldest is 92—to its clinics in San Francisco and Tampa. Patients pay $8,000 to participate, which covers the costs of the plasma, transfusion services, and blood tests.
The patient-funded trial doesn’t target any specific disease—rather, Karmazin hopes to target multiple diseases at once. Some entrants suffer from conditions such as diabetes, heart disease, and Alzheimer’s disease. “A fair number of patients are younger and healthier, and they want to stay that way,” Karmazin says. “And I think that’s easier. In theory, it should be easier to keep someone healthy than to reverse a disease, but it does seem to show a potential for both.”
The effects of aging seemed to have been reversed—although it wasn’t clear that the effect derived just from blood.
Karmazin says the patients report immediate results, starting as soon as the transfusion does. Patients, he says, tell him they feel stronger, that the visibility of their wrinkles decreases. They can immediately resume normal activity afterward, and some report lifting more weight at the gym.
“These results are still preliminary, but they’re showing some really strong changes,” Karmazin says. In the blood tests, he reports seeing three changes in particular. First, there is a decrease in the number of amyloids, which can form plaques implicated in diseases like Alzheimer’s and Parkinson’s. There is also less inflammation, and lower cholesterol—a sign of cardiovascular health.
Although the results are drawing new clients to the trial, Karmazin did not use a placebo or double blind methods, which are both standard practice for medical tests. His studies have also not been peer-reviewed or published. Because of this, scientists in the community have been critical of his work. Even if young blood were proven to posses restorative qualities, would the benefits be worth the potential risks of an unnecessary transfusion?
“The number one thing I’d be concerned about would be the risk of spreading disease,” says Alan Mast, a senior investigator at the Blood Research Institute at the Blood Center of Wisconsin, and an expert on transfusion medicine with the American Society of Hematology. Mast stresses blood transfusions today are safer than they’ve ever been. Still, he says, “To me, it’s a risk. Is it really worth it if you don’t need the transfusion?” Older patients prone to congestive heart failure could also be at risk for volume overload from a transfusion, which could make their heart condition worse.
Epidemiological studies are also contradicting the early reports of transfusion benefits. Gustaf Edgren, a hematologist and associate professor of epidemiology at the Karolinska Institutet in Sweden, studied a large database of blood transfusions and donations in Sweden and concluded that the age of blood donors have no correlation with recipient mortality, the opposite of what you’d expect if young blood had a rejuvenating effect. (The patients he studied had received transfusions they needed to survive.)
Edgren sees Karmazin’s methods, in particular, as “unethical and potentially quite dangerous.” Without any placebo to compare against, Edgren says, “the study is scientifically disinteresting.” He doubts whether Karmazin’s results can be taken seriously since there is no way of knowing if the reported benefits derive from the age of the donor blood, the plasma transfusion itself, or some other variation that was not experimentally controlled.
Edgren says the claims that young-blood transfusions can arrest aging remain premature. “My opinion as a clinician is that humans die much more from disease than from mere old age,” he says. “There’s so much hype surrounding this, that they’re just not advancing the field. It’s not going to do any good for the patients, and it’s not going to do any good for our scientific understanding of aging.”
Edgren is hopeful future research can move toward firmer conclusions by more closely emulating the techniques in the mouse studies. “I’m not saying there couldn’t be such an effect from the young blood also in humans, but to test this we need to design studies for humans that mimic the mouse models much more closely.”
In the meantime, scientists continue to buy and sell millennial blood for injection into the old and hopeful. While we don’t treat young blood as the elusive ambrosia of legend, after centuries of scientific advancement it is remarkable how attached we are to the same story.
Natalie Coleman is an editorial intern at Nautilus.



























