At first, no one looked twice at the new variant. Detected in South Africa in January 2021, the novel coronavirus lineage, called C.1, appeared similar to other variants. It wasn’t spreading much, and there was nothing unexpected about its genome.
But viruses evolve fast. Exceptionally fast. Faster than any other organism on Earth—and the new coronavirus is no exception. Ed Feil, a professor at the University of Bath, studies the evolution of pathogens and recently analyzed the coronavirus’s mutation rate. “SARS-CoV-2 has experienced roughly the same amount of mutational evolutionary change during the pandemic (proportional to genome size), as humans have since Homo habilis first walked the Earth about 2.5 million years ago,” Feil explained in The Conversation.
It’s no surprise that just four months later, when South Africa was suffering through a third wave of COVID-19 caused by the highly transmissible Delta variant, a team tracking the virus began detecting a new version of C.1 with extensive changes in its genome. They soon discovered that the variant, dubbed C.1.2, is more mutated than any other major variant sweeping the world. It contains all the key changes found in Alpha, Beta, Gamma, and Delta, as well as additional worrisome changes currently under investigation, including some associated with an ability to evade the immune system.1
The question of whether anything else happens in the evolution of this virus is moot. It will.
“This version has so many more mutations,” says computational biologist Cathrine Scheepers at the National Institute for Communicable Diseases in Johannesburg, who was part of the team that spotted C.1.2 in May 2021. For now, thankfully, the new variant remains at low levels compared to Delta, says Scheepers, but it has been detected in 10 additional countries. “We’re still monitoring it,” Scheepers adds.
As a science journalist entrenched in daily COVID-19 news, I’ve recently noticed some public health officials forecasting Delta as the last major wave of the pandemic. They tend to qualify their remarks with something akin to “assuming nothing else happens.” We can’t assume that.
SARS-CoV-2 has already morphed to be 40 to 60 percent more transmissible than the Alpha strain, which was already 50 percent more contagious than the original strain, evolving into what some epidemiologists are calling the most infectious disease of our lifetime. The viruses that cause measles and chickenpox transmit slightly more easily than Delta, yet Delta has a faster cycle time, so it whips from one person to another in four days, as opposed at least 10 to 14 days for the other viruses.
The question of whether anything else happens in the evolution of this virus is moot. It will. As long as there are vulnerable populations that can be infected, the virus will transmit, replicate, and mutate, evolving as it spreads. Evolution by natural selection is a law of biology in the same way that gravity is a law of physics; it is a literal force of nature. Continued spread of this virus will lead to further mutation, new variants, more deaths, and an ongoing pandemic.
It’s not that humans aren’t attempting to manage the evolution of this virus. We are. Every day, vaccine makers, researchers, and governments are tracking viral changes, identifying and containing new variants, and attempting to slow the spread. Our species has undertaken the largest vaccination campaign in history, inoculating 3.9 billion people with at least one dose of a COVID-19 vaccine within two years of the initial outbreak.
Yet we’ve been unable to contain this pathogen. SARS-CoV-2, an epitome of evolution by natural selection, is an extraordinary opponent. As humans have countless times before and will again, we underestimate the power of nature at our peril.
Each SARS-CoV-2 genome is made up of 30,000 individual chemical bases, represented as letters, which store instructions for a suite of proteins used to hijack our cells and produce billions of new viral particles.
When a person breathes in the virus, the infamous spike proteins on its surface recognize and attach to proteins on human cells. Although the process of infection begins in our throats and lungs, the virus is capable of attacking cells and systems throughout the body, including the heart, blood vessels, gut, kidneys, and more.
Once attached to a cell, the virus shoots its genome—a single, coiled strand of 30,000 RNA bases—into the interior of the cell. There, viral proteins begin remodeling cellular structures to accommodate mass production of viral parts. Like a factory architect and manager rolled into one, the viral genome leads this multifaceted, coordinated effort to make more virus.
As humans have before and will again, we underestimate the power of nature at our peril.
Each new viral particle carries a freshly minted copy of the viral genome and is primed and ready to infect more cells. As the particles depart the host cell, they trigger a cascade of events that kill it. As it dies, the cell releases signals to the immune system, like highway flares, alerting the body to danger. In some cases, the resulting immune response to those signals causes more harm than help, including severe lung damage and/or widespread hyperinflammation called a cytokine storm.
Now, remember those freshly minted copies of the viral genome? Mistakes occur during the process of copying RNA, such as one chemical base switched for another, or a small chunk of RNA added to or removed from the original sequence. Better known as mutations, these random changes are typically small, like altering the angle of a brushstroke on a painting, but the ramifications can be great. We commonly think of mutations as bad for an organism, such as a mutation on a hemoglobin gene that causes sickle cell disease. But a mutation can also be neutral or beneficial, such as mutations in a gene associated with insulin production that make a person 65 percent less likely to get diabetes, even when they have risk factors like obesity.2
The viral genome gains new mutations, both good and bad, with every infection as new copies of the virus are made. In June, a team at the Weizmann Institute of Science in Israel and collaborators calculated that every time a human is infected with SARS-CoV-2, their body produces between 1 billion and 100 billion copies of the virus. The team also estimated that 0.1 to 1 mutation is likely to occur within the virus’s genome during each infection. If we conservatively agree that each infection adds 0.1 mutations to the viral genome, then among the 425,000 global daily cases, 42,500 mutations occur. That means it’s possible that every one of the 30,000 bases in the coronavirus genome is mutated each day.3
Thankfully, few of those mutations gain a foothold in viral populations. There’s a transmission bottleneck. Even if a mutation occurs within a single infection, that mutation is rarely passed onto another person. According to two recent studies,4,5 the small amount of virus passed on to others is usually identical to the strain that started the infection. Or, as Vaughn Cooper, a microbiologist and director of the Center for Evolutionary Biology and Medicine at the University of Pittsburgh, eloquently describes to me, “The code that went in is usually the one that comes out.”
There is an exception, unfortunately. If the virus spends extended time in a single body—such as in an individual with a weakened immune system, who is unable to clear the virus—it will extensively interact with the human immune system and gain useful mutations to combat it, like going through a bootcamp and coming out stronger. In the past year, for example, scientists have observed SARS-CoV-2 variants gain mutations that change the shape of the spike protein just enough so that protective antibodies—which bind like a lock-and-key to the spike to identify and neutralize it—no longer attach.6
As the virus lingers in one body, new mutations replicate to a point that they are ubiquitous and can be passed to others, slipping through the transmission bottleneck. Evidence suggests the Alpha variant may have first appeared in an immune-compromised individual, and Scheepers believes it was the case for C.1.2 as well.
Still, most mutations that accumulate within a single infection are subject to the transmission bottleneck and will be lost. But not all. The virus has spread so exponentially that even rare events occur, so mutations are gained and passed on. Any mutation that gives the virus a competitive edge to survive and reproduce in its environment—known as its “fitness”—is more likely to be passed on and become a permanent part of the genome. That’s natural selection in action.
Today, SARS-CoV-2 gains about 2 permanent mutations per month in the global population. This virus is not out of tricks yet.
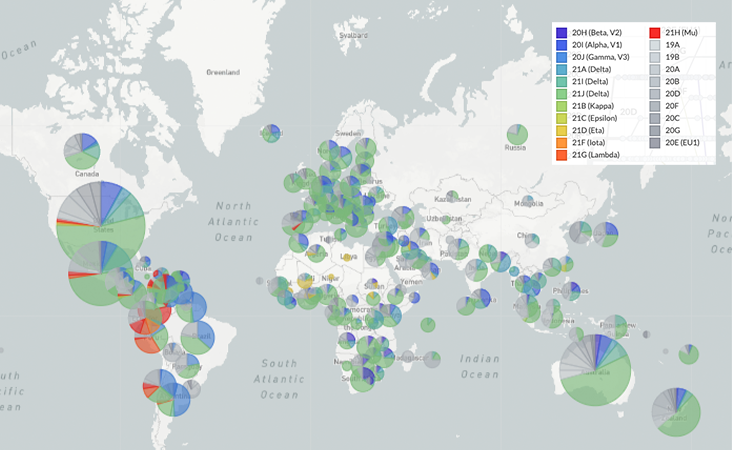
On Jan. 5, 2020, virologist Zhang Yongzhen at the Shanghai Public Health Clinical Center in China uploaded the first genome SARS-CoV-2 sequence onto a public database, despite an order from the Chinese government forbidding the publication of any information about the disease. Zhang’s example initiated a torrent of sharing worldwide. As of late October 2021, the GISAID Initiative, a free genome-sharing platform launched in 2008 for influenza, has accumulated over 4.7 million shared SARS-CoV-2 sequences.
Those millions of shared genome sequences enable scientists to track viral mutations in near real-time, a first in human history. After establishing a system for detecting mutations, scientists began determining, in excruciating molecular detail, what the mutations do. One of the first major SARS-CoV-2 mutations—and the one that accelerated the spread of the virus around the globe—is D614G, sometimes referred to as “Doug.”
In early April 2020, Doug climbed the charts in the United Kingdom, then began a worldwide tour. Researchers found that as soon as this mutation was introduced into a region, it quickly became the most common form of the virus. The mutation itself is an A to G base change at position 23,403 in the viral genome. That change switches out one amino acid (aspartic acid) in the virus’s spike protein for another (glycine). That single swap causes the virus’ receptor binding domains—the bits of the virus that hook onto human cells—to stick more often in an up position and latch onto passing cells.
Trying to guess the next mutation is a fool’s errand. Trying to prevent it is a worthwhile goal.
Doug was a harbinger of things to come. Today’s list of fitness-altering mutations reads like alphabet soup: P681R, L452R, D950N, del144Y, K417N, T1027I, A701V, N501Y, L18F, del242-244, and on. Based on studies conducted around the globe, SARS-CoV-2 mutations are resulting in increased transmissibility of the virus, increased resistance to antibodies (those generated by natural infection and vaccines), and increased severity of disease symptoms.
While some single mutations, like Doug, have clear effects on viral fitness, scientists believe it is primarily combinations of mutations that transform the variants into the juggernauts that they are—especially Delta, which accumulated nine mutations in the spike protein that together enable its wildly increased transmissibility.
Plus, RNA viruses have another method to quickly evolve: They are known for recombination events, in which multiple viruses within a single cell exchange entire sections of RNA, resulting in Frankenstein-like genomes. Now that multiple variants are in circulation, it is possible—even likely, according to some epidemiologists—variants could swap and combine parts into a so-called “super” variant. While recombination events have been detected, they’ve so far been restricted to small clusters of people, and surveillance efforts tracking them are ongoing.
But bear with me, as it’s not all bad news. Over the last 22 months, scientists have identified a silver lining to the virus’s evolution. Yes, natural selection is pushing the virus toward increased viral transmission and immune evasion, but not toward more severe disease in humans. Severe disease, such as a cytokine storm, is a by-product of infection, and does not appear to help the virus transmit or reproduce any better, so evolution does not select for that trait, says Cooper.
Another bit of good evolution news: Viral populations around the globe appear to be settling on similar mutations. At Fred Hutchinson Cancer Research Center in Seattle, Trevor Bedford, Katie Kistler, and colleagues recently used GISAID’s data trove to show that the most successful lineages of the virus gained spike protein mutations associated with improved cell entry, as well as mutations in two other proteins, Nsp6 and ORF7a, implicated in viral replication and evading the innate immune system.7 In many cases, they found that identical mutations appeared independently around the globe, a process called convergent evolution.
If the virus is converging on certain key adaptations, it is becoming more predictable, which may allow scientists to track and combat it with more confidence. “The good news is we’re not seeing entirely new combinations,” says Cooper. “That, hopefully, is calming.”
Trying to guess the next dominant mutation is a fool’s errand, but trying to prevent it is a worthwhile goal being undertaken by vaccine makers and governments around the world.
The only way to slow the evolution of the virus—and prevent additional mutations and new variants—is to slow viral transmission, says Kistler, a molecular biology graduate student at the University of Washington. “Each infection is a chance for more evolution of the virus,” she notes. Whatever we can do to reduce the number of infections—and to take particular care of chronically infected individuals—can help us manage the evolution of SARS-CoV-2.
Our most powerful weapon to slow transmission is vaccination. Different vaccines work in different ways to protect the body against an invader, but the general gist is that a vaccine presents an inactivated form or piece of a virus—something non-infectious—to the body to simulate the immune system against it, like training an army to fight a specific foe.
Pfizer and Moderna’s mRNA vaccines, for example, contain instructions for our cells to produce the virus’s spike protein. After our cells make a bunch of harmless spikes, the body’s immune system notices and attacks those foreign proteins and produces T cells and B cells that stick around to fight that virus if it ever enters the body again.
It will only be through widespread vaccination that we will slow the evolution of this virus.
Besides preventing infection, another perk of vaccination is that if a vaccinated person does become infected, they tend to produce a lower amount of virus in their body and they clear the infection more quickly than an unvaccinated person, giving the virus less time to mutate.
When doctors or scientists mention “escape” variants of the virus, they’re referring to variants with mutations that can avoid (escape) the protection gained from vaccination or previous infection. The Beta variant, for example, carries mutations—including E484K or “Eek”—that enable it to partially dodge the immune system because antibodies are less likely to bind to the spikes. Back in February 2021, South Africa even stopped using the AstraZeneca vaccine after clinical trials showed it did not provide protection against mild-to-moderate COVID-19 caused by the Beta variant.8 The spread of the Delta variant also appears to be partially driven by mutations in the spike protein that allow it to evade the immune system better than the original spike.9
For now, vaccine companies say their approved vaccines are the best protection against all known variants, and most are encouraging booster shots for at-risk groups, as recently approved by the FDA. Further, Pfizer, Moderna, and AstraZeneca are practicing what it would take to quickly produce a vaccine for an escape variant by doing dress rehearsals. They’re making vaccines based on current variants, then running through the workflow of getting them tested and approved. “We want to practice all aspects of executing a strain change … so that if we do see a variant out there that truly escapes vaccine immunity, we’re ready to go fast,” Philip Dormitzer, vice-president and chief scientific officer of viral vaccines and mRNA at Pfizer, told Nature in October.
For any new variant that crops up, Pfizer and partner BioNTech expect to be able to “develop and produce a tailor-made vaccine against that variant in approximately 100 days after a decision to do so,” Kit Longley, a spokesperson for Pfizer, told me.
Humanity is in a race with the virus to vaccinate as many people as possible before the virus can evolve new variants. Most estimates suggest we need 60 to 70 percent immunity across populations to slow or stop transmission. As of late October 2021, 48.7 percent of the world population has received at least one dose of a COVID-19 vaccine, according to vaccination rates estimated by the Our World in Data project at the University of Oxford. That sounds good, right? Pretty close to 60 percent?
Unfortunately, the world is not uniformly vaccinated at that high rate. There is a yawning gap between countries. Wealthier countries have high vaccination rates, and less wealthy countries do not. Overall, only 3 percent of people in low-income countries have received at least one dose, meaning those groups of people are still highly susceptible to infection, and the virus has extensive breathing room to continue to transmit and evolve. PATH, a U.S.-based nonprofit global health organization, calls the rollout of COVID vaccines “a global emergency,” stating that vaccinations for many around the world could be months, even years away.
There are also uneven vaccination rates within single communities, as we’ve seen in the United States. A patchwork network of vaccination—highly vaccinated populations side-by-side with unvaccinated populations—creates a melting pot for the virus to simmer into something stronger, something worse. Here’s the scenario: In unvaccinated pockets of people, high quantities of virus spread. The abundant virus manages to infect a vaccinated person, where it interacts with vaccine-induced antibodies. Novel antibody-evading mutants emerge, then are passed back into an unvaccinated population to increase in number and spread again, this time infecting both vaccinated and unvaccinated populations.
As infections continue, evolution continues. I’m as tired as the next person of wearing a mask, sanitizing, and maintaining distance from others. But it will only be through vaccination and these safety measures—which work against all variants of the coronavirus—that we will slow the evolution of this virus.
Megan Scudellari is a science journalist for national magazines and newspapers. She has been covering the COVID-19 pandemic since March 2020. Follow her on Twitter @Scudellari.
Lead image: Lightspring / Shutterstock
References
1. Scheepers, C., et al. Emergence and phenotypic characterization of C.1.2, a globally detected lineage that rapidly accumulated mutations of concern. medRxiv (2021).
2. Flannick, J., et al. Loss-of-function mutations in SLC30A8 protect against type 2 diabetes. Nature Genetics 46, 357-63 (2014).
3. Sender, R., et al. The total number and mass of SARS-CoV-2 virions. Proceedings of the National Academy of Sciences 118, e2024815118 (2021).
4. Lythgoe, K.A., et al. SARS-CoV-2 within-host diversity and transmission. Science 372, eabg0821 (2021).
5. Braun, K.M., et al. Acute SARS-CoV-2 infections harbor limited within-host diversity and transmit via tight transmission bottlenecks. PLoS Pathogens 17, e1009849 (2021).
6. Yuan, M., et al. Structural and functional ramifications of antigenic drift in recent SARS-CoV-2 variant. Science 373, 818-823 (2021).
7. Kistler, K.E., Huddleston, J., & Bedford, T. Rapid and parallel adaptive mutations in spike S1 drive clade success in SARS-CoV-2. bioRxiv (2021).
8. Madhi, S.A., et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. New England Journal of Medicine 384, 1885-1898 (2021).
9. Mlcochova, P. et al. SARS-CoV-2 B.1.617.2 Delta variant replication and immune evasion. Nature (2021). Retrieved from doi:10.1038/s41586-021-03944-y