For most of our history, wolves have been a menace to humanity. Sharp teeth, raw speed, and pack coordination put us at serious risk. Their howls still send chills down our spines for good reason. But some thousands of years ago, some wolves gradually became used to, and even fond of, humans. Canis lupus became Canis familiaris. Eventually, humans took more active control of the evolutionary trajectory of the wolf, molding its descendants into dachshunds and golden retrievers and great Danes. The offspring of an animal that ate our ancestors now protect us from intruders, return our Frisbees, and dutifully allow our children to ride them like horses.
Some researchers have begun to believe that we can use a similar approach to decrease the risk of infectious disease. After getting a better handle on why pathogens evolve to be harmful in the first place, scientists are beginning to figure out how to coax them towards becoming more benign.
For the better part of the last century, the prevailing dogma asserted that virulent pathogens—those that caused serious harm—were poorly adapted to their hosts. According to the theory, all pathogens would eventually evolve toward benign relationships. After all, what good does it do a parasite to kill its host? Likewise, a bed-ridden host comes in contact with fewer people, limiting the spread of the infectious organism.
Unfortunately for humanity, it seems that that idea missed a key part of the equation: “There is a competition among strains to exploit their own host,” says Giulio De Leo, a researcher in ecological theory at Stanford. The latest research suggests that many pathogens do not necessarily evolve towards harmonious relationships, but instead, take a more selfish approach. If a pathogen can gain an advantage by usurping more resources, replicating more voraciously, or destroying more host cells, it will evolve toward virulence, even at the cost of destroying the host. Pathogens aren’t altruistic; each strain is searching for a fine balance point, which maximizes how aggressively it can reproduce while still maintaining maximum transmission. “There’s a tradeoff,” says De Leo.
With this idea in mind, some scientists are trying to figure out how to manipulate a pathogen’s environment so virulence is not an advantage. In 1991, evolutionary biologist Paul Ewald observed one of the best examples of this sort of manipulation during outbreak in Peru of cholera, which causes potentially life-threatening diarrhea in humans. In areas with poor wastewater treatment, like Peru, cholera was transmitted through fecal matter in dirty water. With this route of transmission open, cholera’s best strategy was to become as virulent as possible. The more diarrhea a bacterium could induce, the better its chances for transmission.
Eventually public health workers cleaned up the Peruvian water supply and caused a turning point in the epidemic. When the bacterium couldn’t travel through the water, it was no longer beneficial for it to cause more diarrhea. The only way for cholera to spread was through personal contact such as touching or sneezing, which required relatively healthy and mobile hosts. Ewald and his team observed that the cholera strains that survived were ones that produced less toxin, causing less diarrhea. By cleaning up the water supply, humans influence the evolution of a bacterium toward lower virulence. They made cholera behave more like the common cold.
“The history of public health interventions is replete with successes and failures,” says Ewald. “The concept of pathogen domestication helps us understand why some of the most dramatic successes were so successful.”
The diphtheria vaccine is an example of one such success. All vaccines work by exposing the immune system to pieces of a pathogen. This allows white blood cells to “learn” what the invader looks like so that when the real thing shows up, the body is ready. The problem with vaccines is that pathogens can change the pieces that the immune system is looking for. The genius of the diphtheria vaccine is that it’s made from the toxins produced by bacteria. If the pathogen wants dodge the vaccine, it has to mutate the very part of itself that makes it harmful. It’s like a metal detector at a concert: it doesn’t look for people, it looks for guns. If a bacterium does try to smuggle guns in the concert, it’s met by a fleet of tough security guards: an army of B-cells that smother it with antibodies before their friends come along and melt the offender’s skin off and digest its insides.
Pathogens aren’t altruistic; each strain is searching for a fine balance point, which maximizes how aggressively it can reproduce while still maintaining maximum transmission.
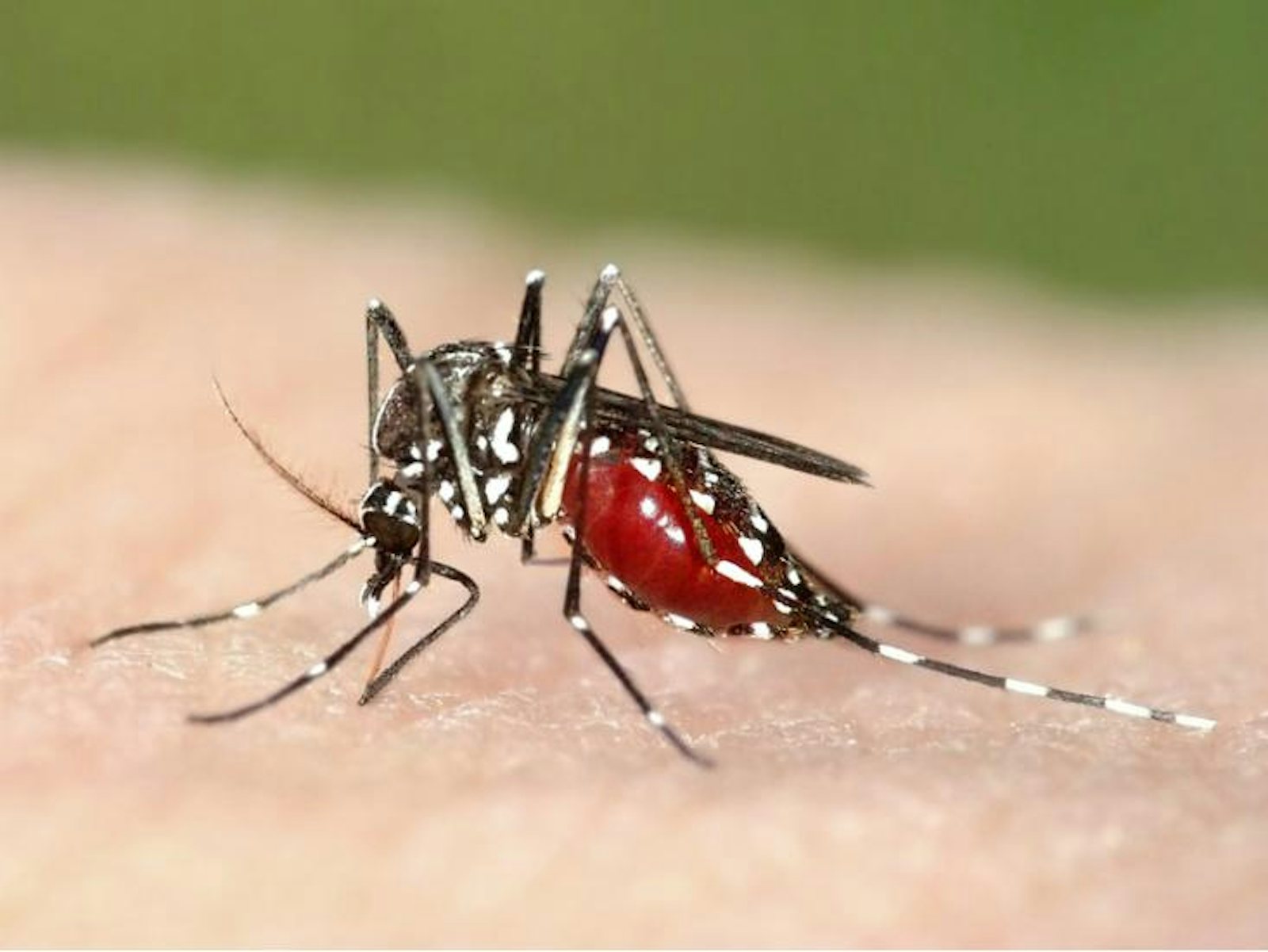
Organisms that don’t rely on human-to-human transmission tend to be more harmful since they don’t need their hosts to be healthy and mobile. Malaria, one of the most deadly diseases today, is caused by a protozoan called Plasmodium, which is transmitted through an insect vector, the mosquito. So far, all efforts to eradicate malaria have been unsuccessful—not only because there are so many mosquitoes and the pathogen is so readily transmitted, but also because Plasmodium reproduces sexually. Unlike bacteria and viruses, which make near-exact copies of themselves when they reproduce, Plasmodium parasites shuffle their genes each generation, giving their offspring more chances to evolve around anti-malarial drugs and potential vaccines.
Ewald thinks that maybe, in the case of malaria, it’s time to try a different approach. The same evolutionary flexibility afforded by sexual reproduction should also make it possible to domesticate Plasmodium. Mosquito-proof housing would cut off the mosquito’s access to anybody infected with a strain of the disease virulent enough to confine its host to bed. If the mosquito’s food sources are restricted to people healthy enough to be outside, walking around, then the only relatively non-virulent strains of Plasmodium can circulate. The idea is to make virulence produce dead-end hosts.
Despite its appealing logic, the concept of pathogen domestication has yet to garner mainstream attention in most academic circles. “If we can try to evolve lower virulence that’s great. But I’d say that in many cases we don’t have a good enough understanding of virulence evolution to be confident that a simple strategy will have the desired effect,” says Read. Tinkering with the evolution of pathogens could even backfire and make more virulent pathogens.
Read’s reserved optimism is typical of the general opinion of Ewald’s theories. Without massive outbreaks of disease, it’s difficult to test or investigate pathogen domestication. Testing pathogen-host dynamics on an evolutionary level is incredibly complex and expensive. Thus far, most of what we know has been gleaned from studying naturally occurring outbreaks like the one in Peru. There’s also the question of timescale. Despite their short generation times, bacteria don’t evolve radical new characteristics over night. Evolution takes time, and while we wait for pathogens to evolve toward being more benign, people are still being infected with dangerous diseases. “Often we need to worry about the short-term issues rather than the long-term evolution of virulent strains,” says Read.
Until now, humanity’s strategy against infectious disease has been one of indiscriminant violence, carried out with broad-spectrum antibiotics, which are increasingly ineffective. We’ve entered an arms race against a nearly infinite number of opponents, some of which have the chance to reinvent and improve themselves every 10 minutes.
Maybe, in the case of infectious disease, violence is not the answer. The same forces that make pathogens flexible enough to dodge our drugs also make them malleable enough to mold to our will. As difficult as it is to change our approach from annihilating to taming the enemy, it may be a necessary change. As long as we’re fighting against evolution, the wolf is always at the door.
A different version of this story appeared on the BU News Service.
David Shultz is an editorial intern at Nautilus.