The weather in Morro Bay, California is too good, the coastline too picturesque, and the wildlife seem to have waltzed straight out of a Disney film. Sea otters play in the waves with their young, herons bask on the beach, and seals stretch their plump bellies in the sun. And yet amid the tranquility of Morro Bay lurks a monster straight from H.P. Lovecraft’s playbook, as slimy as a creature from Sartre’s nightmares. It doesn’t get much more extraterrestrial than this. Two hearts? Tentacles coming out of its head? Four rows of pointed teeth? A vertical smile on its face? But would you call it a smile? Or a face? Why, it’s the hagfish of course.
The writer and Nobel Prize winner John Steinbeck was not a fan of the monster. He found it “disgusting” and “nauseating,” while noting that his close friend, the marine biologist Ed Ricketts, “did not feel this, because the hagfish has certain functions which he found fascinating.” And I find them fascinating too.
Threatened hagfish can bind vast quantities of water into a dense slime in a split second.
The hagfish has certain peculiarities, including its German name, Schleimaal (slime eel), which is misleading, since this elongated creature is not an eel—but neither is it a true fish, as its English name suggests. In fact, hagfish, like the parasitic and similarly unpleasant lamprey, are the last remaining members of the primeval cyclostomata, which translates as “round mouths.” The name is better suited to lampreys, which possess disc-shaped suckermouths with far too many teeth, enabling them to latch on to fish and shred flesh from their flanks. A sensational find confirmed the unique evolutionary journey of cyclostomes.1 It was the first time a fossilized hagfish emerged, 100 million years old and fantastically well preserved, complete with traces of slime, like a “sneeze in stone.” It underlined the close relationship between these creatures and lampreys and proved that they are not primitive ancestors of us vertebrates, as some scientists had previously assumed.
Unlike the lamprey, the hagfish appears to be mostly harmless, living in the depths of the ocean where it feeds largely on carcasses. On land it’s only seen by those who, like me, patrol the streets of Morro Bay looking for a fish wholesaler.
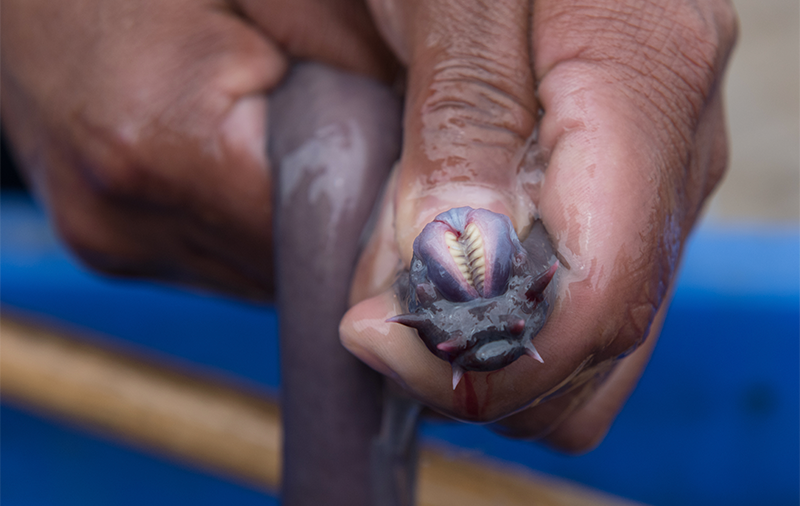
Sandy Winston is one such man, and he takes pity on me on this dull December afternoon just before Christmas and lets me into his yard, where hundreds of hagfish are coiling and contorting in two outsized metal containers which are supplied with a constant flow of water from big hoses. It doesn’t stay that way, since the creatures promptly transform the liquid into a stringy, gelatinous mass. I watch it happen and feel it too, because soon I’m rooting around in the transparent slime, trying to grab a hagfish with both hands.
They’re harder to catch than I’d expected because they never stay still, slipping through my fingers. And then there’s the slime, which is so tough that I can lift it up as a dense fabric. It is so stringy that it creates webbing between my fingers, and so sticky that I won’t be able to wash it off, instead having to rub down my hands with a slime-stiffened old cloth, which Sandy’s colleague Becky gives me without a word. Blonde and quick to laugh, she stands next to me by the container, rooting around in the slime too. She’s responsible for the animals and is now searching for a dead hagfish. “I can smell it,” she says. Why remove a dead hagfish surrounded by carrion-loving members of the same species? “They don’t eat each other,” says Becky.
But neither does the hagfish have many predators. Its loose skin makes it too difficult to catch, and there’s the extremely slimy defense. When threatened, hagfish release extra-long molecules from their skin, stored as space-saving spindles, waiting to be put into action. Then they positively explode, binding vast quantities of water into a dense slime in a split second, forming a suffocating cloud of gel that will even gag a shark. The tens of thousands of fibers in each liter of hagfish slime are long and thin yet durable and elastic, a bit like robust silk or synthetic fibers. And, as entirely natural molecules, they could just show the way toward novel eco-textiles.
But there’s more: The United States Navy is trialing the use of military lab-grade hagfish slime to stop suspicious ships in their tracks without using force. Current tactics involve launching plastic ropes that slow the ship down by getting tangled into its propeller engine but are hard to untangle afterwards. A weapon based on synthetic hagfish slime might snot a suspicious ship in its tracks instead by expanding underwater into a mass of mucus and dissolving later without residue. It would be the modern recreation of a mysterious slime-like sea described in Greek and Roman antiquity. “For over 2,000 years, geographical writings have been haunted by mention of a ‘congealed sea,’ which prevents ships which reach it from sailing any further or makes the journey much more arduous,” wrote the German historian Richard Hennig in 1926. “Mention of this phenomenon appears in the Middle Ages too, in tales of the ‘congealed sea,’ the ‘motionless sea,’ seen from time to time under its Latin name, Morimarusa.”
Who knows how many other unique and potentially useful slimes are out there? If the hagfish is the king of animal goo, its kingdom encompasses all of nature—and every single species within it. Biological slimes are not obscure exceptions, they are the rule, and essential for survival. In all my years of researching this fascinating material, I’ve yet to encounter a slime-free creature and doubt that such a minimalist even exists. Small wonder, as there’s hardly a single evolutionary question to which nature has not found an answer in slime. As Mark Denny of Stanford University wrote in a trailblazing publication in 1989, invertebrates in particular rely on the stuff for movement, communication, reproduction, self-defense, and even food, while jellyfish, comb jellies, and other zooplankton are composed entirely of gelatinous mesoglea.
An all-encompassing medium in life: This is what slime means to microbes as well. But what about us, the so-called higher organisms? We vertebrates, as well as plants are not above this. We use slime in myriad ways too, we’re simply a little less obvious about it. This is a necessity for terrestrial species, because the highly hydrated slime dries out quickly in the air. Any creature using it on a large scale on land will prefer to hide it away inside its body or as a plant in soil, where water loss can be more readily controlled. The only openly slimy surfaces we humans display are the eyes. They are covered in thin films of mucus which are protected from dehydration by a lipid layer. Slime’s mostly secretive nature might be the reason why it escaped our notice for so long as an essential and sophisticated substance.
The U.S. Navy is trialing the use of military lab-grade hagfish slime to stop suspicious ships in their tracks.
And what is slime in the first place? It’s the default term for unknown but sluggish fluids or creepily soft solids. It’s a thing in between and a feeling and a description of materials, but there is not one prototypical slime. Depending on origin and function, it hides behind a multitude of pseudonyms like gel, biofilm, mucilage, glycocalyx, but also in ecological communities like biological soil crusts, or in phenomena like marine snow.
But how to unravel the differences and commonalities here? Most gelatinous substances are lumped together under the label of “slime,” even in scientific publications, without their molecular inner lives receiving much illumination at all. Or at least it was that way a few years ago: Today, ever more researchers working on specific slimes are connecting with colleagues to join the dots. An international collaboration under the lead of Adam Braunschweig at City University of New York seeks to investigate different animal mucuses and use their designs to develop new technologies. It’s a worthy goal since these slimes are, as they state in a publication, “remarkably diverse and include lubricants, wet adhesives, protective barriers, and mineralizing agents.”2 And the brand-new discipline even has a name: “mucomics.”
But even if specific slimes can be pinned down by their structure and functions, which definition should we take for all kinds of biological slimes? The answer may be as slippery as the substances themselves, but they share at least some important characteristics—in regard to their components, structure, behavior, and function. And it might just be easier to unravel this from the outside in, starting with the functions. Varied as biological slimes are, they act mainly as lubricants, glues, and selective barriers. There are other functions like hydration or mineralization, but these can often be subsumed into the major categories which themselves are not entirely distinct.
I’ve yet to encounter a slime-free creature and doubt that such a minimalist even exists.
So far there are only a few species whose slimy repertoire we know about and have researched in any detail. Snails, for instance, can crawl along just as easily as they hang from a surface simply by secreting a different slimy glue. And they coat inner surfaces like the digestive tract with slime as a barrier—just as humans and many other organisms do. But what if there aren’t any tissues or surfaces inside a body to protect, when one cell is the whole organism? Microbes are the original and possibly the most proficient slimers of all. They bunch together and build themselves a gooey city or biofilm wherever there is some water and surface to attach to. Microbial slimes in the environment are ubiquitous enough to affect habitats from deserts to coasts by gluing sand, sediment, and other substrates together, often on interfaces between air, land, and water.
Now let’s consider behavior: How can slimes act as lubricants, glues, and flexible barriers? This is due to their viscoelasticity, their ability to behave like a fluid and a solid at the same time. In many cases, organisms can adjust this behavior, fine-tuning the fluidity, the stickiness, and density of their slimes, which makes them particularly adaptable to changing needs. How hydrogels behave depends on how long and how intensively certain forces are acting upon them. This is mainly what makes them so varied and adaptable as lubricants, adhesives, and barriers—despite their being little more than water. The characteristic sluggish fluidity or viscosity depends on the material’s inner structure and components.
“Slime is little more than stiff water,” according to the German microbiologist Hans-Curt Flemming. Part of that rigidity is thanks to a three-dimensional framework which binds the water—holds it in molecular chains. In other words: The water wants to flow but is kept on a short, if elastic, lead by the molecular framework, which accounts for the more solid behavior. This network is formed of polymers, long-chain molecules which are cross-linked. They are unique in their ability to bind together extraordinary quantities of water, at least when it comes to high-functioning slimes produced by biological organisms.
While science is only now getting a grip on the study of slippery elusive slime, it is not the first time slimes have aroused human curiosity and been exploited for human purposes.
The yellow slime of the banded dye-murex snail turns to deep violet in bright sunshine. Thousands upon thousands of these molluscs were killed in ancient Rome, and later also in the royal households of Europe, for extravagant gowns in imperial or royal purple. The common piddock Pholas dactylus had the similar misfortune of being extraordinarily prized. These creatures grind holes in stone using their elongated shells, concealing themselves inside for life. Despite their nifty burrows they were found by the Romans, and Pliny writes of night-time Pholas feasts during which guests’ mouths, hands, and clothing would glow in the dark because they had been sprayed with the molluscs’ bioluminescent slime. It looks like we might just ooze our way back into a future where we use and appreciate nature’s gels in even more surprising—and hopefully more subtle—ways. ![]()
Susanne Wedlich studied biology and political science in Munich and has worked as a writer in Boston and Singapore. She is currently a freelance science journalist for Der Spiegel, National Geographic, FAZ, and others. She lives in Munich. She is the author of Slime: A Natural History.
Excerpted from Slime: A Natural History, by Susanne Wedlich; published by Melville House, 2023.
Lead image: Simia Attentive / Shutterstock
References
1. Miyashita, T., et al. Hagfish from the Cretaceous Tethys Sea and a reconciliation of the morphological-molecular conflict in early vertebrate phylogeny. Biological Sciences 116, 2146-2151 (2019).
2. Cerullo, A.R., et al. Comparative animal mucomics: Inspiration for functional materials from ubiquitous and understudied biopolymers. ACS Biomaterials Science & Engineering 6, 5377-5398 (2020).