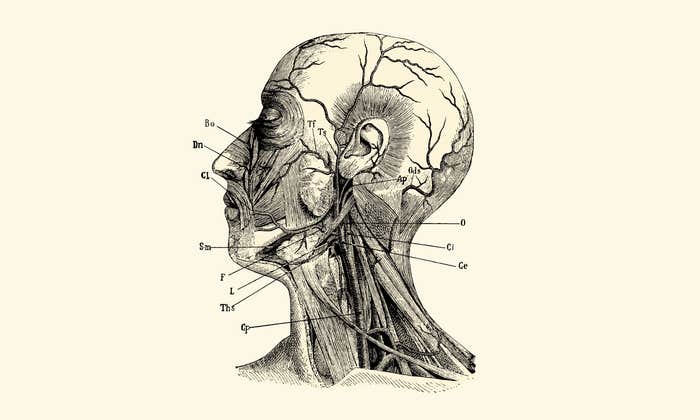
For the loved ones we’ve all lost, for the families that we can still save, let’s make America the country that cures cancer once and for all.
—President Barack Obama, State of the Union Address (2016)
Patrick Soon-Shiong wants to turn cancer treatment upside down. On January 12, Soon-Shiong and a consortium of industry, government, and academia announced the launch of the Cancer MoonShot 2020, an ambitious program aiming to replace a long history of blunt trial-and-error treatment with what amounts to a training regimen for the body’s own immune system. That system, Soon-Shiong argues, is perfectly adept at finding and eliminating cancer with exquisite precision—if it can recognize the mutated cells in the first place. Helping it to do so could represent a powerful new treatment for the disease, akin to a flu vaccine.
Soon-Shiong has hit home runs before. This past July, one of his firms underwent the highest-value biotech IPO in history. A cancer drug he developed, called Abraxane, is approved to fight breast, lung, and pancreatic cancers in more than 40 countries. Soon-Shiong’s path from medical school in South Africa through residency in Canada, to UCLA professor, NASA researcher and corporate CEO has given him the bird’s-eye view necessary to take on a project this ambitious, as well as the resources to marshal the world-class computing and genome-sequencing facilities that it requires.
When I sat down with him after the MoonShot announcement, I found him enthralled by the power and aesthetics of newly emerging cancer science, and deeply optimistic about near-term outcomes. This, it seems, is an exciting time to tackle cancer anew.
What’s wrong with how we treat cancer today?
We handle cancer based on empirical trial and error and imperfect information. We now have more information than we have ever had in the history of science. And that data reinforces a belief I have always had as a young doctor, which is that we are born with inherent protective mechanisms that fight cancer, infection, and infectious diseases. The way our body reacts when we are infected by a virus, or tear an Achilles, involves the same biology as when we fight cancer.
Have we been too simplistic in our thinking about cancer?
Yes, I think that’s why we’ve been losing the war. As physicians we’re trained to be reductionist. We rigidly follow protocol. But life is not that way. Cancer is not linear—it is completely non-linear. It lives in the science of chaos. There’s no single point of control. You need to attack it in a non-linear fashion across time and space, monitoring it and truly dancing with it. I know this sounds philosophical and silly and esoteric but it’s not. If you biopsy a patient with breast cancer twice in the same day, once in the breast and once in the lymph node, you can get cancer cells with different sequences. Even if you biopsy two different points in the breast, the sequence can be different. This heterogeneity has really only come to light recently. Which breaks all these reductionist assumptions, because which target are you hitting and what made you choose it? Is it just because you biopsied here instead of there? You’re whacking a mole, but you have no idea which one you’re whacking. You whack this one, this other one wakens. The only chance we have, in my opinion, is to do what I call micro killing and macro killing at the same time. Micro killing meaning you go after these little targets, maybe even using a little chemotherapy. And macro killing meaning either surgery, radiation, or immunotherapy.
Cancer represents a breaking of the contract with the human body.
How do you see cancer?
I see it as a bunch of rare diseases. Every cancer has its own molecular profile. There are many different sub-types just in lung cancer. They all hijack the immune system, tricking your body into believing they are not there. If we can teach your immune system to outsmart cancer by realizing that these cells in your body need to be killed, and if we can mobilize our immune system, then we have a completely new shot at treating cancer. The flip side of this hypothesis is, why would you give yourself chemotherapy at the highest doses, which wipes out the immune system? That’s what we’ve been doing for 40 years. I’m not saying you shouldn’t give yourself chemotherapy at all—but why would you give yourself such a high dose that you actually wipe out whatever protective mechanisms that you already have? Let’s understand the biological complexity of cancer, and use that understanding and trick the cancer to kill itself. And I think we have figured out a way to do that.
Is cancer a normal part of life?
Yes, I think cancer is actually a part of your physiological normal self. There’s a process called apoptosis by which your normal cells die, like the autumn leaves going brown. Cancer is actually not just an out-of-control growth—it’s a prevention of death, meaning the cells refuse to die when they should. You are right now as we’re doing this interview producing cancer cells in your body. What normally happens with these abnormal mutations is that your immune system’s natural killer cells are recognizing them and killing them. I don’t think mankind could have been born without natural killer cells having evolved to protect us against infection and cancer. So cancer is the flipside of regenerative medicine and stem cells. You know for many years people didn’t believe a cancer stem cell even existed. But it does—it’s just your normal stem cells gone amuck. Cancer represents a breaking of the contract with the human body.
Do the cells that you’re talking about have a kind of intelligence?
If you watch the motion of cells interacting with each other, you realize that they are exquisitely intelligent. They perform what I call the dance of proteins. The natural killer cell itself has something like 30,000 receptors on it. It is just one cell but it actually sees what’s around it, and turns on, turns off, activates, de-activates. And you know the amazing thing about the immune system is that it’s incredibly beautiful in its orchestration. Once you understand that orchestration, the simplicity of using that for the treatment of cancer is so amazing.
What is the Cancer MoonShot 2020?
The Cancer MoonShot 2020 is a project that seeks to exploit the body’s own immune system to fight cancer. Around 2015 Vice President Biden called me about his son’s brain cancer, and I got involved with some of the diagnostics. His son passed away in May of this year. By October I had written a two-page white paper talking about accelerating cancer immunotherapy using genomic sequencing and big data. That white paper became the mission statement of the MoonShot. My job as a physician, a surgeon, a cancer oncologist, immunologist, NASA ex-scientist, and former CEO is to orchestrate all of this. We are pursuing a very, very ambitious program. I’m not saying we’re going to cure cancer by 2020, but maybe we’ll be able to activate the body’s T cells to fight it. Just this week we’re going to present the first neoepitope targeting antibody, which will target mutant cancer proteins. We have treated metastatic colon cancer patients that have failed every line of chemotherapy and every line of standard therapy. All they got is this one antibody as an injection. Thirty percent are alive today. Some patients have been alive for two years. The average survival for these patients should have been five months. The promise of this program is not some hypothetical promise, but it is here now, tangible but very complex.
We can interrogate cells in real time in a way we’ve never ever been able to do by using CRISPR technology.
How do modern genetics fit into the MoonShot?
In 2003 people said we’ve solved cancer because we’ve solved the human genome, but that was completely naïve because it’s not the gene that’s important at all. It’s the output from the gene. What we called junk DNA actually turns out to control gene expression. So it was a naïve, almost arrogant assumption that just by solving the genome we’re going to know what we’re doing. We needed to actually take this junk DNA, 3 billion base pairs that control 20,000 genes, and understand it. To do that we needed to what’s called whole-genome sequencing. Which we’ve now done for the first time. Then we found that it’s not the gene, stupid—it’s the protein that the genes make. These 20,000 genes and this junk DNA control something called transcription, which involves 200,000 rRNA (ribosomal DNA) molecules. Then this rDNA is involved in making 10 million proteins through 10,000 different pathways. So has the science actually evolved to be able to take 3 billion base pairs times 20,000 genes times 200,000 rRNA molecules times 10,000 pathways, and find the pathway that actually causes the cancer? Yes. We are there. But, again we had this arrogance of saying that’s all we need. We have this other thing called passenger genes, which we thought we weren’t interested in. It turns out those passenger genes are the ones that are tricking the body into believing that mutant cancer proteins are not foreign. What if we can isolate those mutants and bring them into the body in the form of a vaccine, like a flu vaccine? Then we can train the body’s T cells to recognize them and go after them. That is the approach we’ve tried with the colon cancer patients.
Is gene editing technology also relevant?
We understand more about mechanisms at the attomolar level and the cellular level than ever. We can interrogate cells in real time in a way we’ve never ever been able to do by using CRISPR technology, which lets us turn cells on and turn cells off and actually test our hypothesis in the test tube. The ability to measure 123 little biomarkers just in the immune system has been demonstrated. We’re learning more about the mechanism of the interaction of proteins than ever before.
How important is big data to the MoonShot?
It’s one of the weapons but it’s information that you actually use in real time to test hypotheses. What frustrates me most when people talk of big data is that they’re really talking about retrospective registries—the stuff that’s going on at research institutions and even at the National Institutes of Health. All these big data registries look at claims data, backward in time. I’m talking about real world data that I’m capturing now dynamically, treating you, knowing that my treatments are actually affecting the relevant mutation. That’s big data. That’s real world data. That’s the data we’re talking about.
How did you get interested in science?
We grew up in South Africa without TV, so we’d listen to radio and we’d read. There were magazines called Knowledge and Look and Learn, with pictures of these cells and descriptions of how they worked, and I think I was 13 when I said okay that’s what I want to do. I was trained at a school called the Chinese High School—you couldn’t go to a white school and you couldn’t go to a black school, you went to a Chinese high school. Our science teacher was a priest who went through World War I who got terribly ill in my last year or second-to-last year of high school. There was no teacher for about six months. And I was nominated to teach the class. So I read these science textbooks and taught my own class science lessons for a while.
What was your medical education like?
I was trained as a doctor in South Africa. It was a six-year training course and one-year internship, and at the end of that there was no specialty training. I got a full generalist training, including deep physiology, deep pathology, deep microbiology, deep pediatrics, and internal medicine. We had to end up having delivered 100 babies. So the exposure that I got to this breadth of medicine was amazing. I then had the good fortune to come to Canada, where I earned a Master’s at night studying protein-protein interactions, while I was doing my surgical residency. My focus was on the gastric inhibitory polypeptide and its role in managing the pancreas in patients with diabetes. Then I get recruited to UCLA, where I was thrown into the cauldron of being a young resident in the department of surgery, where you’re up all night, you’re doing surgery. Then I got bored. I started out doing these minor surgical procedures but decided I wanted to do the most difficult procedure, which at that time was a thing called the Whipple. A Whipple is a procedure in a patient with pancreatic cancer, in which you basically remove the pancreas, the stomach, a piece of the liver, and the bowels. You remove 80 percent of the area around the pancreas and you have to hook it all up again. That excited me. Then as an assistant professor of surgery I went off to train to do pancreas transplants. I did UCLA’s first two pancreas transplants. If you look at my evolution of generalist to scientist to surgeon to surgical oncologist to pancreas transplants it looks discordant, but to me it was a continuation of understanding biology under different circumstances.
What got you interested in cancer specifically?
The first two pancreas transplant patients at UCLA did fabulously, except they both rejected their transplant. Pancreas transplant rejections are the most frightening thing, because you’ve hooked the pancreas to the bladder. When the organ rejects, port-wine blood pours out the uretary catheter. I said to myself, “wow, do I really believe this is the right thing to do to a patient?” Which led to me tell my chairman that I’m going to shut down the program of which I’m a director. I decided that I needed to understand regenerative medicine, where I can take cells representing only 2 percent of the gland, one thumbnail full, put them into a micro encapsulation, and prevent rejection. Then I could do a transplant with a single needle. Then I got exposed to the Jet Propulsion Lab at NASA, where they were planning to create stem cells for astronauts on Mars, and got involved with that program. I got interested in the immune system because I was trying to induce tolerance, to make your body believe that this cell I’m going to give you as a diabetic patient is actually your cell, even though it came from a pig, so please don’t reject it. Out of that work came two things. One was the invention of Abraxane, a cancer drug which is also the nation’s first protein-based nanoparticle. The other was the realization that cancer cells have figured out how to induce tolerance, to tell your body “don’t eat me because I’m actually you.” So the irony is that the first part of my career was to induce tolerance for transplants, and the second part was to break tolerance to actually tell the body to kill cancer cells.
More information is available at www.cancermoonshot2020.org.