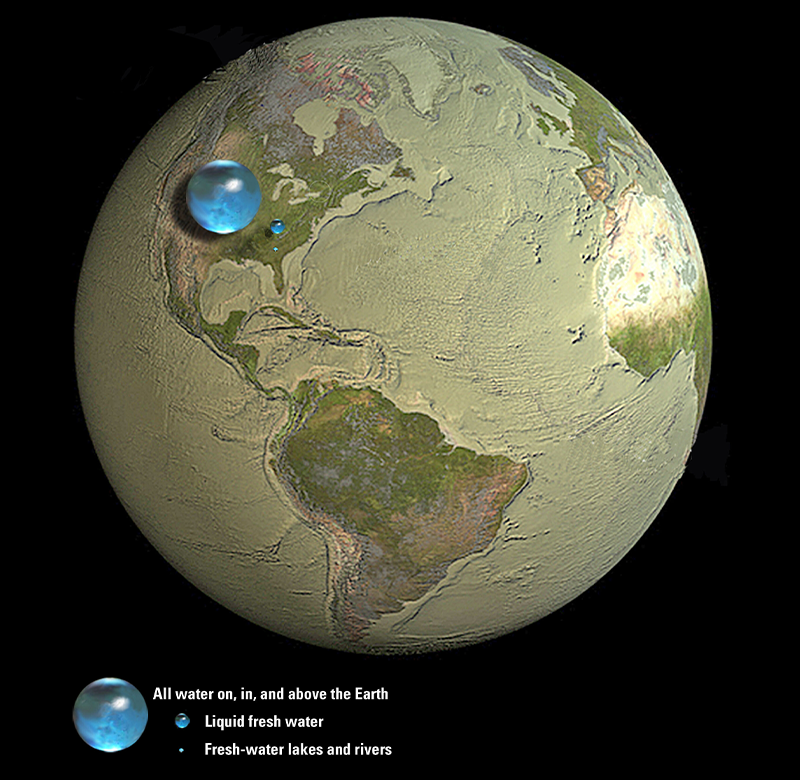
When Carl Sagan famously called Earth the “pale blue dot,” he was judging a book by its cover. Even though three quarters of our planet’s surface is covered by oceans, our planet is actually very dry. Water makes up about one part in a thousand of Earth’s mass (most of it is simply rock and iron). I say “about” because we don’t know exactly how much water is trapped in Earth’s interior. Estimates range from less than one “ocean”—defined to represent the sum of all of Earth’s surface water (oceans, lakes, glaciers, and so on)—to more than 10. Regardless of the exact water budget, Earth is about 10 times drier than crackers (which typically contain about 2 percent water).
A gripping new story about the origins of water is emerging from analyses of meteorites. The old story was that water-rich asteroids from afar “delivered” all of Earth’s water during our planet’s formation. New results show that, contrary to this narrative, most of Earth’s water was sourced locally, much closer to the sun.
Meteorites are rocky leftovers or remnants from the time when the planets were forming that managed to survive the fall to Earth. They might have broken off as fragments from any rocky body after a powerful impact. Most meteorites are fragments of asteroids, whose orbits were slowly perturbed over millions of years to cross Earth’s path and crash down. Sometimes, chunks of rock from the moon and Mars, launched into space after an impact, could land on Earth, pulled in by its gravity, as lunar or Martian meteorites. Like spilled coffee grounds and cream drops, these chunks are evidence of what went into the cup long after it had been brewed and drunk.
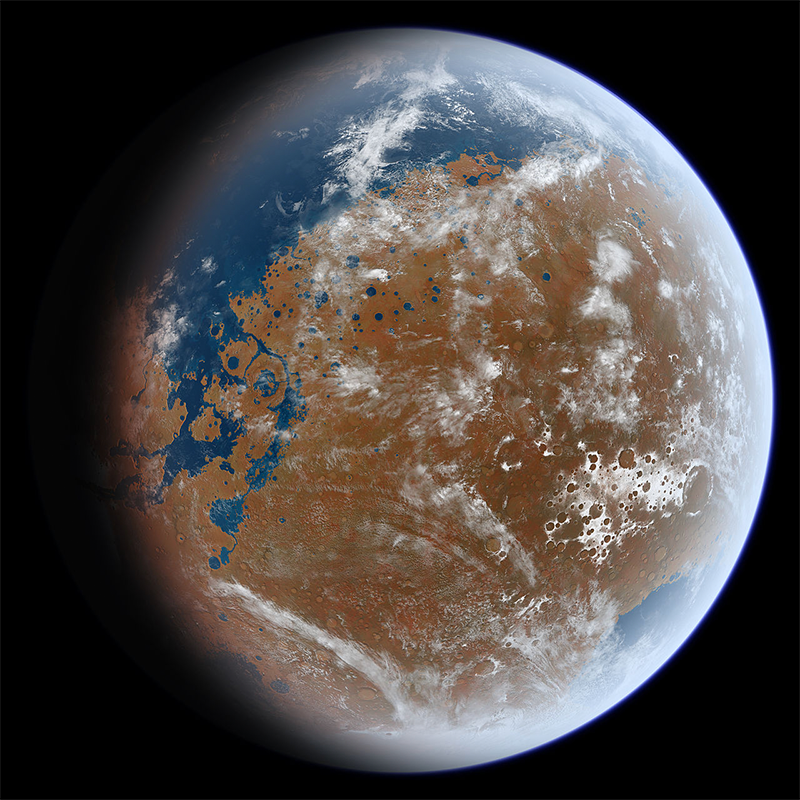
Mars may have had rivers and oceans in its distant past before the water was lost to space.
In graduate school, I found meteorites endlessly confusing. There were so many different classes and types based on their appearance, how much heat and pressure morphed them, and their compositions. But over the past decade, the study of meteorites has become much simpler.
Now, meteorites fall into just two classes: CC and NC—also called “carbonaceous” and “non-carbonaceous.” CCs come from the outer part of the asteroid belt, which runs between Mars and Jupiter, and NCs from the inner part of the belt. When scientists measure the isotopes of different atoms in meteorites, they fall into just two clusters corresponding to these classes. In my mind, this meteorite “dichotomy” is the biggest discovery in planetary science of the past decade.
The most important aspect of the meteorite dichotomy is that no meteorites lie in between the CC and NC clusters. The CC meteorites are fragments of C-type asteroids, which often contain a lot of water. Meanwhile, the NC meteorites are fragments of different types of asteroids, notably S-types, which are drier.
The existence of two classes of meteorites tells us that there were two different reservoirs of planet-forming material in the sun’s planet-forming disk. NC asteroids (which include S-types) and CC asteroids (which include C-types) are next-door neighbors in the present-day asteroid belt. The weird thing is that, given their isotopic differences, they could not have formed right next to each other, because if they had there would be meteorites in between the two classes. Does that mean that the two classes of meteorites formed at different times, or in different places?
Earth is about 10 times drier than crackers.
We can infer the ages of meteorites using radioactive clocks, and it turns out that the ages of CC and NC meteorites overlap. During the first 3 to 4 million years of the solar system, both CC and NC objects were actively forming. This means that the CC and NC objects formed in different places in the solar system. Exactly what kept the two classes separate is uncertain, but it may have been the growing Jupiter, as it launched density waves in the sun’s gaseous planet-forming disk that blocked the inward flow of large dust grains, the building blocks of asteroids and comets. Regardless of the exact mechanism at play, the research I’ve done with colleagues suggests that the CCs formed past Jupiter and were later implanted into the asteroid belt, whereas the NCs were born closer to the sun.1
What fraction of Earth is made of CC versus NC material? We can measure the same isotopes in Earth rocks that are measured for meteorites. For rock-building elements like chromium and molybdenum, Earth is much closer to NC meteorites than to CC ones. So, the rocky parts of Earth (meaning, most of the planet) is mostly made of NC-like rocky stuff from the inner solar system. Earth was built mostly from NCs with just a splash of CCs. For decades, that splash is thought to have represented the source of all of our planet’s water. But new meteorite measurements suggest that this splash was not the main source.
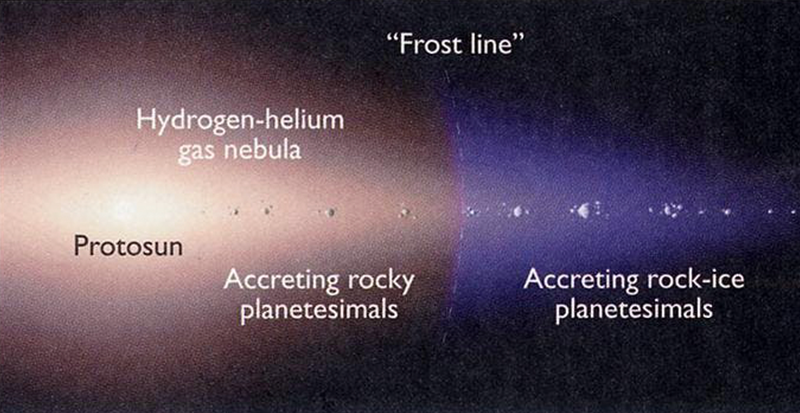
The building blocks of the planets—mountain-sized “planetesimals,” and also the parent bodies of meteorites—range in composition. The NC planetesimals are thought to have dryly formed close to the sun, whereas the CC planetesimals formed farther out, past the “frost line,” in a cold enough region that they could incorporate water ice. The “frost line” in a planet-forming disk is the distance beyond which it is cold enough for water to condense as ice. Closer to the star, water exists as vapor.
It has long been known that the CC planetesimals provide a decent match to Earth’s water in terms of hydrogen isotopes (specifically, the deuterium to hydrogen ratio, or D/H). So, for the past 20 years it has been thought that a sprinkling of CC-like planetesimals delivered water to Earth while it was forming. This apparent requirement for water delivery from objects from beyond the frost line has had a foundational influence on the construction of models for how the planets were assembled. The change in our thinking about the origin of Earth’s water has the potential to destabilize long-standing formation models. Yet, surprisingly, our best current models not only survive this change in our understanding of the origins of Earth’s water but are actually reinforced.
The key assumption of almost all models to date is that the NC planetesimals that built the Earth were dry. The meteorites that provide the closest match to Earth’s composition is a subclass called Enstatite chondrites. A 2020 Science paper by University of Lorraine cosmochemist Laurette Piani and colleagues showed that Enstatite chondrites contain more water than previously thought—enough to potentially explain all of it, with no sprinkling of water from farther out.2 They also measured the isotopes of another element, nitrogen. They found that neither Enstatite chondrites or CC meteorites can explain both Earth’s water and nitrogen at the same time.
In 2022, two studies in the journal Icarus—one led by exoplanet scientist Paul Savage,3 the other led by geochemist Theodor Steller4—measured meteorite isotopes of still another element, zinc. It’s a moderately volatile element—it can exist as a solid at higher temperatures than hydrogen, carbon, nitrogen, and oxygen, but it vaporizes much more readily than elements like iron. These studies show that matching Earth’s volatiles requires a mixture of Enstatites and CCs. When you measure the isotopes of Earth’s volatile elements, you find that Earth lies in between the NC and CC clusters. About two-thirds of Earth’s water (and its zinc and nitrogen) seem to have come from Enstatite-like planetesimals, and the other third from CC planetesimals.
Since CCs are a lot wetter than NCs, everything fits nicely together if Earth was built from about 95 percent NC material and about 5 percent CC. This is the same ratio that matches Earth’s rocky elements, just like the coffee with a splash of cream—the only difference being that we now realize that most of Earth’s water came from the coffee, not the cream.
A 2023 Icarus study led by Thorsten Kleine, co-director of the Max Planck Institute for Solar System Research, uses the same techniques to figure out the origin of Mars’ water.5 Even though Mars cannot currently support liquid water on its surface, because of its low atmospheric pressure, it may have had rivers and oceans in its distant past before the water was lost to space. Kleine and his colleagues measured zinc isotopes in Martian meteorites and found them to be different from Earth, instead falling right in the NC cluster. This means that almost all of Mars’ water is homegrown, from an NC source like Enstatite chondrites or S-type asteroids.
Most of the latest models naturally form rocky planets like Earth and Mars that are mostly made up of local (NC or Enstatite) planetesimals, with a splash of CC planetesimals that were kicked into the inner solar system by the growing (and perhaps migrating) giant planets. When two drastically different approaches (meteorite measurements and computer simulations) get the same answer, it’s reassuring. This gives us a sense of convergence on an underlying “truth,” kind of like finally figuring out the recipe for that oh-so-good mocha latte.
Sagan would have been excited about the idea of using meteorites to figure out the origin of Earth’s and Mars’ water. Even though the precious liquid only makes up a tiny fraction of our planet’s mass, its presence on the surface is of cosmic importance—not only because of its essential role in life, but also because its outer blue sheen serves as a sign to extraterrestrial gazers looking Earthward from light-years away: Lots of things are possible on a pale blue dot. ![]()
Sean Raymond is an American astrophysicist working at the Bordeaux Astrophysical Laboratory in France. He also writes a blog at the interface of science and fiction (planetplanet.net) and recently published a book of astronomy poems.
Lead image: Tasnuva Elahi; with images by Vadim Sadovski and design36 / Shutterstock
References
1. Raymond, S.N. & Izidoro, A. Origin of water in the inner solar system: Planetesimals scattered inward during Jupiter and Saturn’s rapid gas accretion. Icarus 297, 134-148 (2017).
2. Piani, L., et al. Earth’s water may have been inherited from material similar to enstatite chondrite meteorites. Science 369, 1110-1113 (2020).
3. Savage, P.S., Moynier, F., & Boyet, M. Zinc isotope anomalies in primitive meteorites identify the other solar system as an important source of Earth’s volatile inventory. Icarus 386, 115172 (2022).
4. Steller, T., Burkhardt, C,, Yang, C., & Kleine, T. Nucleosynthetic zinc isotope anomalies reveal a dual origin of terrestrial volatiles. Icarus 386, 115171 (2022).
5. Kleine, T., Steller, T., Burkhardt, C., & Nimmo, F. An inner solar system origin of volatile elements in Mars. Icarus 397, 115519 (2023).




























