A year ago, Joe Biden launched his “cancer moonshot,” a major national push to improve the prevention, detection, and treatment of cancer, a plan that was widely recognized to be incremental. “I believe that we need an absolute national commitment to end cancer as we know it,” Biden said while he was on his tour to cancer centers at Penn and Duke University. “I’m not naïve. I didn’t think we could ‘end cancer.’ I’m not looking for a silver bullet. There is none.” Many thought the “moonshot” risked casting the solution to cancer as an engineering problem.
In his op-ed in the New York Times last year, “We Won’t Cure Cancer,” Jarle Breivik, professor of medicine at the University of Oslo, emphasized the government plan was typical of “huge programs, stocked with technology and experts, to solve presumably intractable problems”—but cancer is as much an organic and ecological issue as an engineering problem with bugs or glitches to be worked out. “Cancer isn’t space travel,” Breivik wrote. “The growing cancer epidemic is not a problem that medical science is about to solve.”
Microsoft flatly stated that it would “solve” cancer by 2026.
Part of the emphasis of cancer as a technology- or startup-challenge owes something to Silicon Valley entrepreneurs who grew up in a world under the promise that big data and software engineering could solve any problem. Facebook cofounder Sean Parker, through his 501c3 non-profit Sean Parker Foundation, promised $250 million to fight cancer, while retaining the patent rights to research projects he funds, part of a new model of free market philanthropy. At a panel at the Vanity Fair New Establishment Summit titled “Hacking Cancer,” Parker spoke of himself as a “technologist” who wanted to make “big bold bets,” while addressing “systemic inefficiencies” in science, such as standardizing protocols and consolidating clinical trials among multiple hospitals, to harness big data. At the same time, as medical research proceeds in small incremental steps, large-scale government funding—in combination with an increasing competitive landscape to patent and market new technologies and drugs—suggests it is the public who is being asked to make the “big bold bets” to subsidize the drug industry.

The theory of cancer as a logic problem, whereby cellular circuits go haywire and enable a cell to turn cancerous, has been the standard paradigm in cancer genetics. In the past decade, scientists have been looking not only to deduce the logic of molecular changes that can enable a cell to become a cancer, but have begun using targeted screens, employing such weaponry as the gene modification tool Crispr-Cas9, to disable each gene in a cancer cell one at a time and decipher a logic that can stop a cancer cell. But the impulse that disruptive technologies employed by software engineers can be applied to biology, as an analog to a machine or computer with bugs which can be hacked or solved—suggested in “hacking cancer”—is deeply engrained. The problem goes back centuries.
In 1747, French enlightenment thinker Julien Offray de La Mettrie published “L’homme Machine,” or “Man, a Machine.” The philosopher of science Karl Popper noted later that the “theory of evolution gave the problem an even sharper edge.” Meanwhile, adherents to the view of biology as mere clockwork grew. The “doctrine that man is a machine has perhaps more defenders than before among physicists, biologists and philosophers,” Popper observed, “especially in the form of the thesis that man is a computer.” The reason this view is popular with Silicon Valley and computer companies is that technologists sell “solutions,” which can become attractive if they eliminate a problem in a market space. But if all life, including cancer cells, continues to exploit niches, no solutions from technologists will be final.
Cancer cells are not simply a disorder or breakdown in a mechanism, but an organism going on a full-tilt offensive, using multiple, often shifting strategies to produce and use molecular fuel, win resources, and evade the immune system. If so, then the rules of the game may change—these insights suggest that the war on cancer may be endless. Still, we can get better at treating it as an evolving entity within the context on its ecology, through the idea of “living drugs,” such as engineering the body’s own immune cells to sense and mobilize an attack on cancer.
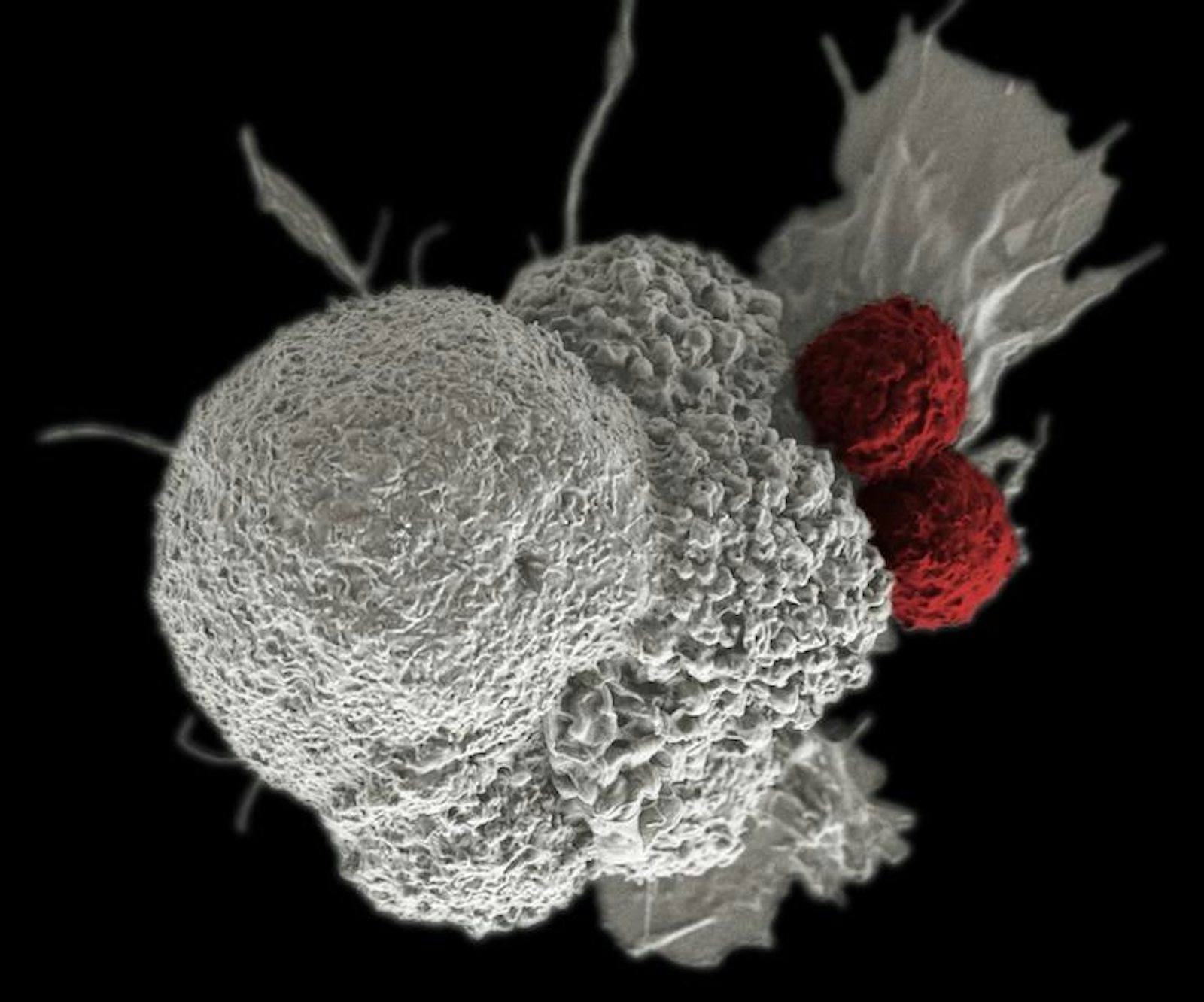
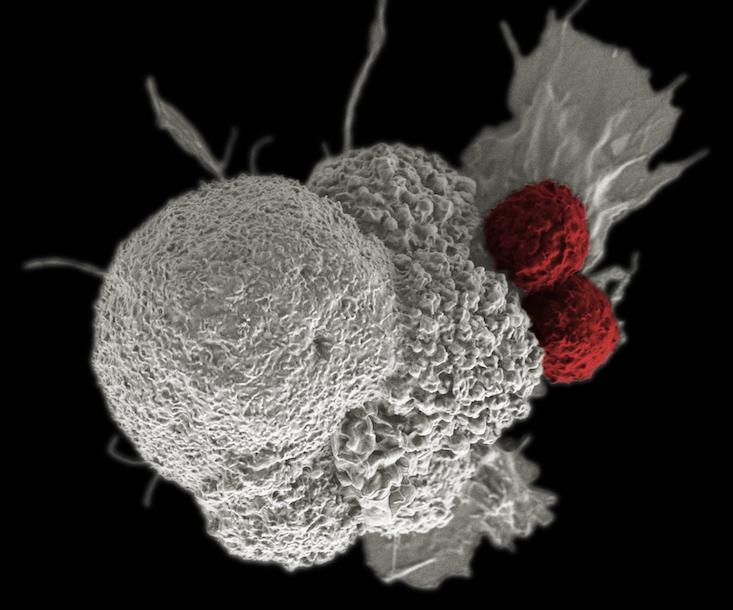
Parker backed Penn scientists in a bid to use the gene modification system Crispr-Cas9 to modify immune system T-cells, rugged soldiers of the immune system which otherwise depend on the precision guidance of antibodies to hone in on their targets, to attach to blood cell cancers. Immunotherapies such as this can involve adding bits of code that build new receptors on the surface of T-cells, which enable them to latch onto markers on the surface of cancer cells, such as CD19 or NY-ESO-1. A number of companies, such as Juno, Kite, and Novartis, are all working on engineering T-cells to fight solid state tumors in organs, stationary masses of cancer that are trickier to fight since they remain burrowed in our bodies and wrap themselves in sheaths of connective tissue called stroma. They represent a target widely perceived as a goldmine among drug makers. Immune-based strategies can also make use of antibodies to inhibit checkpoint blockade mechanisms, receptors on the surface of cells that cancer cells use to temper or turn off an immune system response, or novel strategies that use viruses to tip off the whereabouts of cancer. However, if cancer cells evolve, the need to evolve new treatments may amount to an endless arms race. If our cells are not done evolving then neither is cancer.
Cancer cells can make ad hoc use of environmental cues.
But the theory of cancer as a logic problem, rather than an evolving entity in the space and time of ecology, persists. Scientists continue to believe that they can deduce the formula of genes that enable a cell to become a cancer, or a formula of genes that can be interrupted to stop a cancer. Indeed, research into the genetics that enables a cell to turn cancerous has been downplayed in favor of investigating the genes that can be disabled to kill a cancer cell. For instance, an initiative at the Broad Institute works to experimentally deactivate each gene, one-by-one, in hundreds of cancer cell lines, such as blood, immune, bone, pancreatic, and breast cells grown in a lab. This winter, the Broad will begin working with I.B.M. Watson Health to deactivate genes in cancer cell lines in combination with small molecule drugs—synthetic compounds which can typically be taken in pill form—to identify secondary drug targets that work together as a treatment protocol. But in evolution there is no definitive model for how a cell may or may not function, and the mechanics of evolution will be worked out in perpetuity. We will get better at treatment, but may never cure cancer.
Yet Microsoft flatly stated that it would “solve” cancer by 2026. Jasmin Fisher, a senior researcher at Microsoft and associate professor at Cambridge University, later walked back some of those ringing pronouncements to me, admitting they were misleading. Fisher said Microsoft’s work involves at least three strategies. In the first, scientists create networks of “nodes” that represent the amount of product expressed by each gene in a cell and “edges” which represent the connection or interaction between any two genes. Whether a node is on or off is simply given a binary value of 0 or 1. Or, the value of a node can be put on a continuous scale. Analysts then “build a logic, spell out a set of rules,” Fisher said. Turning off a combination of nodes in the computer model may be predicted to stop a process such as proliferation of a cancer. These are network models based on fixed input on a computer.
In a second approach, the transitions of a cell through a series of states to cancer, or its acquisition of a resistance to a small molecule drug, is analyzed and used to predict a series of genes which are essential to any transition. Microsoft and AstraZeneca showed leukemia may evolve to gain resistance to specific drugs such as PIM inhibitor, and through these approaches identified a secondary target to deactivate in combination: AKT, a gene that builds a protein called a kinase, which relays a signal to the cell to initiate processes such as cell proliferation. In a third “hybrid” approach, Microsoft uses radiographs and X-rays to show how a tumor is progressing in space and time, to model how changes to genes may predict the shape and migration of a solid tumor to “inform dynamic programs which capture the logic and why a tumor may expand,” Fisher said.
Computational approaches such as these tend to assume data is complete and cell circuits operate in a closed-system, as an analog to a computer circuit, ignoring that cancer cells can evolve in countless ways to exploit a growth advantage. Problematically, a shift in understanding cancer as a three-dimensional problem has risen of late, as we are finding that walls, which separate genetic neighborhoods, can break down, resulting in interplay between growth signals and hundreds of genes that turn on cell growth and its energy use. Cancer cells can use various shapeshifting tricks to turn into metastatic forms that travel to other sites in the body, miraculous alterations that can have nothing to do with alterations in the genetic code. In part, to this end of extending the investigation of cancer to the alterations in the “software code” of a cancer cell, the American Society of Clinical Oncology has released a new journal called JCO Precision Oncology, which may include investigations which contrast the genetic and epigenetic alterations in a single patient, so-called N of 1 studies.
But the problem of cancer may even go beyond the cancer cell, its circuitry and shapeshifting tricks. Cancer cells can make ad hoc use of environmental cues. Caloric restriction reduces recurrence, for example, and cytokine IL6 works to block apoptosis, which explains why aspirin can reduce cancer risk.
Breivik told me the problem of cancer is ecological, extending beyond the circuits of the cell. He said, “[Are systemic approaches] the solution to the problem of cancer? Absolutely no. Systemic approaches are important, but we need to consider that the system of cancer goes far beyond the biochemistry of cancer cells. Cancer relates to our fundamental constitution as multicellular organisms, our limited lifespan, the epidemiology of the aging population, socioeconomics and the future of society. Those who believe that the problem of cancer can be solved by killing or reprogramming cancer cells need to take a step back from the molecular technicalities and take a look at the bigger picture.”
Jim Kozubek is a data scientist living in Cambridge, Massachusetts, and the author of Modern Prometheus: Editing the Human Genome with CRISPR-Cas9.
This article was originally published in Cancer Focus in March 2017.