One of the criteria that make a Scotch a Scotch is that it’s made with Scottish water. That means that a clever connoisseur should be able to tell whether her drink is authentic by tracing the source of its H2O molecules. But how? After it’s collected and filtered of impurities, water is water, right?
Not exactly. The elements hydrogen and oxygen appear in nature in various forms, called isotopes, which differ in neutron number. By measuring the relative abundance of these isotopes in a water sample using a technique called mass spectrometry, scientists can determine its “isotope ratio.” And as it turns out, the isotope ratio of water varies dramatically from place to place.
Scientists first noticed this in the 1950s, when they started collecting freshwater samples from around the world, says Gabe Bowen, a geochemist at the University of Utah. What’s more, he says, researchers realized isotope ratios “varied in predictable ways.” The proportion of heavier water isotopes decreased at higher latitudes, higher elevations, and further from the coasts.
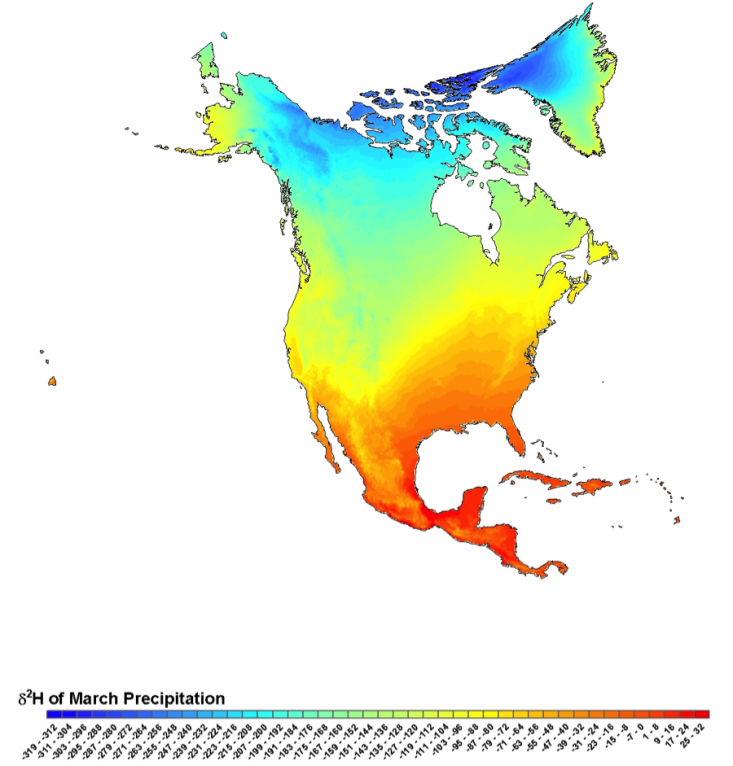
By the 1990s, the U.S. Geological Survey had amassed a collection of nearly 5,000 isotope measurements of river water and precipitation from around the country. When hydrologist Carol Kendall and her colleague Tyler Coplen analyzed these data, they confirmed the large-scale pattern of water isotopes across the U.S. Then, they used their measurements to create a map of the isotopes of water at every location in the country. The result, published in 2001, was a detailed national isoscape—a topographic map that showed isotope ratios instead of elevation.
Since then, researchers like Bowen have created computer-generated isoscapes for different elements and diverse geographic regions, from a single watershed to the entire planet, using knowledge about atmospheric circulation, rainfall patterns, and other physical processes to make the best estimates for unmeasured areas. Hydrologists and forensic scientists can then use these maps to trace water molecules back to their origins—even after they’ve been absorbed by the cells of plants and animals, for example, or turned into distilled alcohol.
One promising application of isoscapes involves the study of water cycles and movement. By comparing isotopes in groundwater samples with precipitation isoscapes, researchers can determine where and when the world’s aquifers get recharged. And in a 2014 study, Stephen Good, at Oregon State University, and his colleagues used isotopic differences between tap water and local precipitation to determine where people in the American West get their water. (You might think we would already know this information, but in fact, the federal government hasn’t tallied up Western water transfers since the 1980s, and there’s no comprehensive database of municipal water sources.)
Then there’s whisky. In 2012, researchers in Scotland reported that the isotope ratios of imitation scotch whiskies did not match those of true Scottish origin, suggesting they hailed from somewhere else. Scientists have similarly used water isotope ratios to suss out the geographic origin of grapes in vintage West Coast wines, and to aid in the identification of murder victims and ancient human remains using bone or hair samples.
Isoscapes have perhaps been most widely embraced by ecologists, who use them to track the movements of migrating animals. “How animals that migrate separate across the planet is one of the great mysteries,” says Peter Marra, the director of the Smithsonian Migratory Bird Center. While scientists know the general patterns of most migrating species, they don’t know the precise paths of individuals or groups—knowledge that’s critical “to understanding everything from their ecology, to their evolution, to doing effective conservation,” Marra says. GPS trackers are helpful, but they can be expensive and are too bulky for small birds and insects.
Isotope measurements offer an alternative. In summer, migratory birds like the pied flycatcher and the American redstart molt and grow a new batch of feathers, which bear the isotopic signature of their location. When a bird arrives at its wintering grounds, researchers like Marra pluck a sample. Its water isotope ratio tells the scientists what latitude the bird came from. (In 2009, Marra used this approach to determine that the birds sucked into the engines of US Airways Flight 1549—forcing it to make an emergency landing in the Hudson River—were migratory geese from Canada, not local geese from New York.)
Isoscapes aren’t perfect locators. Because isotope ratios don’t vary much from east to west, for instance, they rarely provide a solid longitudinal coordinate. But, Marra says, they are as essential to forensic science as are fingerprints at a crime scene. “It’s truly detective work,” he says.
Julia Rosen is a freelance journalist based in Portland, Oregon. Find her on Twitter @ScienceJulia.
This article originally appeared on our blog, Facts So Romantic, in January 2016.































