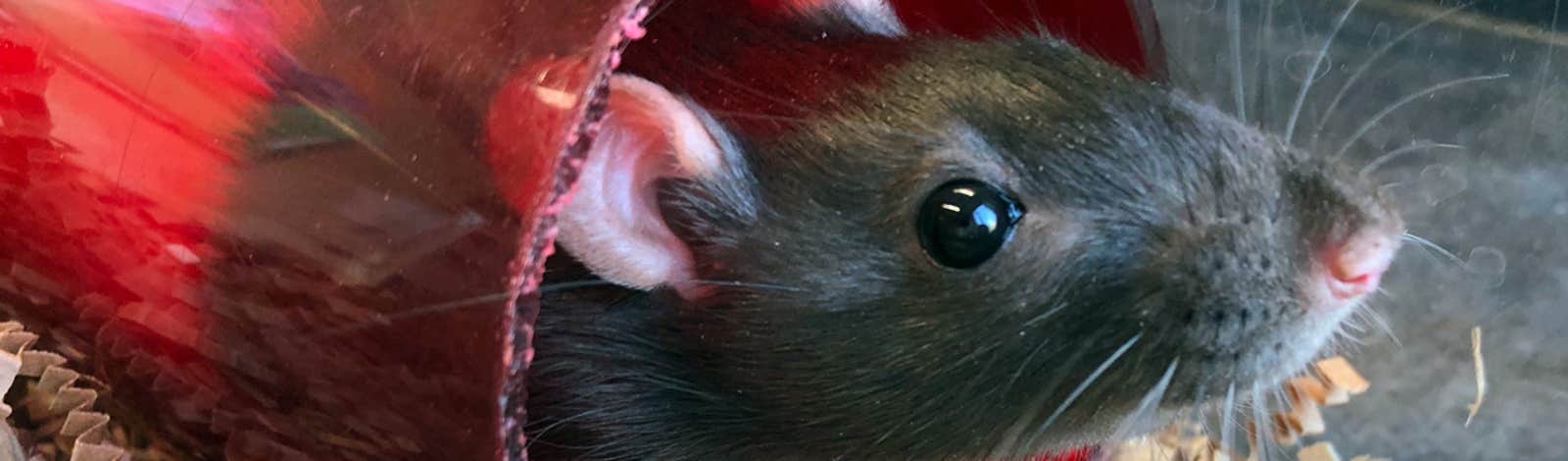
The rat sat still in the middle of her cage, moving only in response to my touch, and even then only as if in slow-motion. My subject, GRat66, was a few months old, and except for her long bare tail, fit neatly into my palm a few minutes earlier, when I injected a few drops of a potent opiate under her skin, near the belly. Now, her beady black eyes bulged as she faded into an opiate stupor.
I was preparing to implant minuscule electrodes into the rat’s brain. The opiate would serve as an analgesic before, during, and after the surgery. It was the fall of 2018 and I was hoping the results of the surgery would help answer some questions that had been tormenting me as I embarked on the sixth year of my Ph.D. in neuroscience. How do the parts of the brain controlling movement interact with those responsible for visual sensation? Why do neurons in the visual areas jolt to action when an animal moves, even in the dark?
I placed GRat66 into an anesthesia-induction chamber, a small plastic box connected to a vaporizer that dispenses the anesthetic, isoflurane. After she was sufficiently knocked out (I pinched her toes to make sure the withdrawal reflex was gone), I placed her into a stereotax, which serves to hold the head steadily in place and provides a flow of isoflurane into her body throughout the operation. I then injected a half a milliliter of lidocaine under the rat’s scalp, which swelled with the local anesthetic’s volume. Dressing myself in a sterile gown, face-mask, and gloves, I proceeded to cut across the length of GRat66’s scalp, exposing the thick ivory-colored skull underneath the pink and bloody skin and shiny white fat.

Peering through a microscope, I drilled a small hole in the skull using a dental drill and carefully peeled back the membrane covering the brain. I positioned the electrodes—wires ten times thinner than a human hair—over one of the visual areas and lowered them slowly into the brain. I then secured the electrodes with glue and closed the wound. At the end of the four-hour procedure, the top of the rat’s head was replaced by a chef’s hat full of electronics that would transmit data from the brain to my computer via a thin tether. I added some finishing touches of glue, squeezing the viscous off-white cement onto the margin between the scalp and the implant, and turned off the anesthetic, letting GRat66 wake up slowly over the following hour.
Scientists do basic research for the sake of knowledge. When reminded that that knowledge has costs, they usually offer a utilitarian argument: The benefits to humanity outweigh the harm done to animals, whose lives have less value than human ones. The picture gets more complicated on the level of individual experiments: If basic science is done for the sake of knowledge, and we don’t know how informative or useful the outcome of an individual animal experiment will be, how can we justify doing it?
Isn’t freedom more valuable than painlessness, even to a rat?
Research on animals has delivered many benefits to humanity. It has given us anesthesia, antibiotics (penicillin was discovered in petri dishes, but its properties and safety were verified in mice), vaccination, and therapies for Parkinson’s disease. The neurotransmitter serotonin, the indirect target of many antidepressants, was found in animal brains by the biochemist Betty Twarog, in 1953. Diphenhydramine, a muscle-relaxing agent initially discovered in research on dogs, was later shown to quell allergy symptoms (brand names include Benadryl), and still later was modified into Prozac.
Scientists try to use previously acquired knowledge and models rather than animals in their studies whenever possible. But our understanding of brains is so rudimentary that attempting to gain more knowledge without studying actual brains would be like trying to solve a crossword puzzle in an unknown foreign language. We won’t be able to produce treatments for bodies if we don’t know how those bodies work.
Still, I’m torn. Keeping sentient beings confined, operating on and killing them, is difficult for me, and doesn’t square with my love of animals. I know a miraculous solution to my quandary is unlikely, but I hope there might be something I can do to ensure the moral balance of my actions leans toward the good. Then, last year, I stumbled on an intriguing project at the Massachusetts Institute of Technology Media Lab called “Reducing Suffering in Laboratory Animals.” Led by geneticist Kevin Esvelt, this research promised to “develop strains of mice that experience far less pain and suffering than current animals.” I sought Esvelt out, curious if his work could help resolve my dilemma.
Esvelt greeted me at his office at the MIT Media Lab on a humid fall afternoon. He is the principle investigator of the Sculpting Evolution group, a lab that uses the latest in molecular and genetic tools to understand evolutionary processes. Currently, Esvelt is creating genetically modified mice that are insensitive to physical pain. A trim 36-year-old with blond hair, Esvelt was dressed in a blue button-down shirt, dark pants, and sandals (no socks). His intense blue-eyed gaze betrayed what I diagnosed as hyper-caffeination. “I’m actually very concerned about animal well-being in general,” he told me. “We engineer mice all the time. Why on Earth do we not genetically engineer mice so it is easier to turn off their perception of pain and thereby reduce suffering?”
Esvelt was inspired by Geoffrey Woods, a geneticist at Cambridge University in the United Kingdom, who has studied rare cases of people insensitive to physical pain. In a 2006 paper, Woods and an international team of researchers reported genotyping results from three Pakistani families with pain-insensitive children. The scientists homed in on the families after someone in the local medical community observed the behavior of a 10-year-old boy, who performed in the streets by putting knives through his arms. He died before his 14th birthday, “after jumping off a house roof,” they wrote in their paper, published in Nature.
After sequencing the genomes of several children from these families, the scientists found a mutation in SCN9A, the gene that encodes the sodium channel Nav1.7, a protein pore that allows charged sodium ions to flow into a neuron, charging it like a battery. Creatures with mutations of SCN9A are normal in almost every respect: They have reasonable intelligence, they can see, hear, coordinate their movements, and feel touch. If someone sticks a pin through their skin, they will feel the mechanical displacement (they might report that something is touching them), but won’t have any idea that the sensation is painful or harmful.
I drilled a small hole in the skull and carefully peeled back the membrane covering the brain.
While the relationship between genes and perception is mind-numbingly complex in most cases, mutations of SCN9A have clear effects. “You knock out this particular receptor and you don’t feel any mechanical pain whatsoever,” Esvelt told me. “We have human testimony to that. That’s the one we know we can do. And we could have done this, frankly, a long time ago.”
While a pain-insensitive lab mouse would be unlikely to jump off a building, having no idea what pain is throughout its life would be a maladaptive trait. To get around this problem, Esvelt and his graduate student Devora Najjar, who joined us in Esvelt’s office later, decided to harness novel genetic tricks that would enable them to induce a knockout of SCN9A whenever they choose, essentially putting a switch on pain sensitivity. This could be done by adding a specific molecule to the mice’s diet, which would prevent neurons from making the sodium channel Nav1.7 for as long as the mice eat the laced chow; after finishing the experiments, the researchers could put the animals back on the normal diet, allowing their genetic machinery to start making Nav1.7 as before.
It’s “important that when the mice grow up, they develop normal pain-sensing behaviors because it prevents them from developing bad habits like chewing on their tongues or getting into fights,” Esvelt said. “You want them to grow up experiencing pain normally and then turn it off just before you’re going to do the experiment.”
I asked Esvelt if having painless mice might, paradoxically, lead to more suffering. A mouse that can’t feel pain might earn less respect from researchers. Najjar, a fast-talking Long Islander, echoed my concern. “People might do more unethical experimentation with an animal that can’t feel any pain,” she said. To avoid this, she proposed leveraging the existing protections that lab animals enjoy; the idea would be to have these painless mice remain categorized as if they were normal animals, except that instead of opiates, they would receive their analgesia via Esvelt’s genetic engineering.
While we now understand pain well enough to turn it off, I left MIT wondering if the painless mouse project missed some of the point of what constitutes suffering. My rats wouldn’t feel a whole lot of pain throughout my experiments, thanks to the opiates I give them before and after surgery, and yet it’s hard to believe they don’t suffer. They spend their entire lives in a shoebox-sized cage, in solitary confinement for a large chunk of that time. They have toys, shelter, and unlimited food. Those benefits, though, don’t seem to make up for their imprisonment and invasive surgery. Isn’t freedom more valuable than painlessness, even to a rat?
That people care about lab animal well-being is heartening, and the fact that the animals have any protections at all is not trivial. It wasn’t always so. Like much of human history, the history of animal experimentation is one of gruesome cruelty. In the pursuit of knowledge, entertainment, or profit, humans have inflicted unimaginable suffering upon billions of animals over centuries.
Human curiosity about animal physiology led the ancient Greeks and Romans to perform vivisections—surgery on awake, kicking, squealing animals. Galen of Pergamum, a Greek physician living in Rome, described his vivisections around AD 177 in a volume called On Anatomical Procedures. For the purpose of brain or spinal cord surgery, he wrote, “you must procure either a pig or a goat.” A pig or goat was preferable to an ape because “you avoid seeing the unpleasing expression of an ape when it is vivisected.” Next, Galen advised, stretch the animal out on a board with holes for straps and secure its limbs; then, procure a large strong knife. “Every cut that you impose should travel in a straight line,” Galen wrote, “and the cut should without pity or compassion penetrate into the deep tissues in order that with a single stroke you may lay free and uncovered the skull of the animal.” After peeling the scalp away and cutting through the skull, you are ready to start your experiment. “You can first of all press down upon the brain on each one of its four ventricles, and observe what derangements have afflicted the animal.” Thus, in the crudest possible terms, the study of the brain was born. In a way, that is how neuroscience is still conducted today: Observe the brain, manipulate it, and see what happens to behavior as a result.
With the rise of Christianity, which preached that God made animals for humans’ use, attitudes toward animals grew bleak. French philosopher Rene Descartes wrote that responses to painful stimuli in animals are merely reflexes, and that animals are not capable of any internal experience because they don’t have souls. To this vacuous view, Voltaire delivered a devastating response about a century later. “Barbarians seize this dog, which in friendship surpasses man so prodigiously,” Voltaire wrote in his Philosophical Dictionary, in 1764. They “nail it on a table, and dissect it alive in order to show the mesenteric veins,” only to “discover in it all the same organs of feeling that are in yourself … Has nature arranged all the means of feeling in this animal, so that it may not feel?” Modern science has validated the great thinker’s rhetorical question, providing an overwhelming multitude of evidence that animals, from mice to macaques, robins to ravens, do feel pain and can suffer, physically and emotionally.
By the late 19th century, some of the experiments done on animals were done under anesthesia, which was discovered and refined in the first half of that century. But it took another hundred years before people started caring about the well-being of animals outside the surgical context. In 1966, the United States Congress passed the Laboratory Animal Welfare Act, which required licensing of labs and animal dealers, inspections of facilities by the U.S. Department of Agriculture, and sufficient postoperative analgesia to be given to animals. Later instantiations of the Welfare Act have established institutional animal care and usage committees that consist of veterinarians, laypeople, and others tasked with ensuring the best possible care of lab animals.
I don’t believe that I have an inalienable right to do research on animals.
As laws began to change, so too did public concern for animal suffering. The biggest bombshell was the publication of Peter Singer’s Animal Liberation in 1975. The book contains nightmarish descriptions of cruel experiments (such as the ones that induce learned helplessness in dogs by delivering inescapable electric shocks), and offers a bleak view of the scientific enterprise. “Among the tens of millions of experiments performed, only a few can possibly be regarded as contributing to important medical research,” Singer wrote. “Experiments serving no direct and urgent purpose should stop immediately, and in the remaining fields of research, we should, whenever possible, seek to replace experiments that involve animals with alternative methods that do not.”
I had heard much praise for the book in my college days, but hadn’t read it until last year. I was startled by Singer’s dismissal of the utility of basic science and wondered if his views had evolved in the more than 40 years since the book was published. I was curious too what Singer would make of Esvelt’s painless mice, and so I called him.
“I’m not going to deny that some experiments on animals have yielded benefits for humans,” Singer offered, cautiously. Over the phone, I immediately recognized the dry, dispassionate tone that made the arguments of Animal Liberation so devastating. Singer admitted that if an animal experiment could “cure some major disease that causes an immense amount of suffering,” and it was shown there was no other way to reach that cure, and the animals wouldn’t suffer in the experiment, “I would be prepared to say, ‘OK, the use of animals was justifiable under those conditions.’”
But what about basic biomedical research and the quest for knowledge? Would animal experiments be justified in that case? “If we’re talking about basic research rather than something that’s curing a disease, then it’s much harder to answer that question,” he said. “Maybe eventually basic research will have some sort of spinoff in terms of helping us to overcome some condition we have. But from my perspective, simply gaining knowledge for its own sake does not justify inflicting significant amounts of suffering on animals. You need to tell how this could ultimately lead to some important breakthroughs which would be good for sentient beings, humans or animals.”
Singer was more positive about Esvelt’s painless mice. “Given that mice are going to be used in situations that would cause them physical pain, would it be good to genetically engineer mice who are insensitive to physical pain? If you put it that way, then I think the answer is pretty clearly yes,” he said. He cautioned that Esvelt’s painless mice could still be upset, depressed, unhappy, frightened, or anxious. Plus, experimenters might feel it’s OK to do experiments that would be unjustifiable in normal mice, not recognizing that Esvelt’s genetically modified mice could experience other kinds of trauma. “So it’s possible that there’s actually more total suffering going on than there would have been if the painless mice didn’t exist at all.”
I was uneasy with Singer’s reluctance to allow basic research to proceed if we don’t know what its impact will be. Allowing only “promising” experiments to proceed misses the fact that by its nature basic research is unpredictable, making it nearly impossible to know which experiments will yield useful results. Only in hindsight do some experiments look more promising than others.
Animal Liberation characterized scientists as oblivious and caring little for animal wellbeing, but this was not how I had thought of myself or my fellow researchers. I needed a different perspective, so I returned to the place my graduate education started, Harvard Medical School, wondering if a seasoned scientist could help me make sense of what had made me uneasy about Singer’s philosophy. I sought out Rick Born, a professor of neurobiology, who has been working on the primate visual system since the early 1990s, asking how neurons in the visual cortex process incoming stimuli and are influenced by the vast connections from higher-level cortical areas.
Born is known among graduate students as a dedicated mentor, not to mention a cycling enthusiast and open-ocean swimmer. I visited him at the Medical School’s Department of Neurobiology on a frosty morning in November. Entering the building that houses the department requires a Harvard ID, but primate labs exercise extra security for fear of would-be animal liberators. As I waited by the lab’s locked front door, the fit 56-year-old with sparkling silver hair floated down a long corridor to let me in.
We chatted in Born’s office, which he shares with his bicycle. He got right to the point. “I don’t believe that I have an inalienable right to do research on animals—it’s something that I believe is justified by the knowledge that we gain and the benefits to society,” he said. Born was personally motivated, he told me, by family and friends who suffered and had died from neurodegenerative diseases.
She won’t encounter a lot of physical pain, but neither will she experience the joy of parenting or bonding with a mate.
Born’s own work on the primate visual cortex centers on basic scientific questions but also has applications for brain-machine interfaces and neural prosthetics. His team is testing a micro-coil device that delivers information into the brain via electrical signals; rhesus macaques, the monkeys Born works with, are one of the few species with the “high resolution vision necessary to test whether such devices could substitute for natural vision,” he explained.
“Almost everyone I know who does basic biological research loves animals,” Born said. “And part of the reason we do this research is because we’re fascinated by animals, fascinated by how brains have evolved, or fascinated with how different brains and different organisms solve the same problems. We are curious about these animals, we love them. Almost everyone I know has pets.” But the fact remains that such curiosity, in the form of basic research, has costs. “We clearly do harm to the animals,” Born continued. “We deprive them of their freedom. We do surgical procedures on them.” And while the standard of care in primate surgeries in the lab is on par with human procedures, “anyone who’s had surgeries knows that it’s not the most pleasant thing in the world.”
And therein lies the conundrum: While modern technology has greatly reduced painful experiences of many lab animals, does that allow scientists to use them as we please? “I don’t believe that there’s a philosophical a priori answer to say whether we can do this or not,” Born said. “It’s a value judgment on the part of society. And it’s a discussion that society needs to have with itself.”
I left Born’s office and walked down the corridor, following a technician wheeling a cart full of apple slices and grapes. As he squeezed through the door into the primate facility, I caught a glimpse of the macaques jumping up and down in their wireframe cages, hooting and shrieking excitedly at the prospect of an afternoon snack.
Basic science is a gamble. You do experiments without knowing what will pan out and what won’t. During my Ph.D., my efforts have amounted to an interesting scientific result, which is that there is a lot more information about movement in the visual parts of a rat’s brain than was previously known. I think that data is worth the animal lives because every bit of knowledge firms up our foundation of basic brain processes. It’s possible my data may one day provide foundational knowledge to scientists trying to cure a disease or engineers creating visual prosthetics.
Still, I can’t deny that I feel uneasy. Genetically engineering rodents to feel no pain probably isn’t the answer. Who is that painlessness for? The animals or us? Maybe society will have that discussion with itself and one day decide that animal experimentation should be outlawed, or perhaps strike a better balance between animal care and inquisitive basic research. For now, I can’t let my emotions interfere with my work. I have to be clear-eyed and focused to make sure the animals receive the best care possible during the experiments. I try to minimize their suffering in whatever way I can.
As for GRat66, it is not likely she will encounter a whole lot of physical pain in her lifetime, but neither will she experience freedom, the joy of parenting, or bonding with a mate. The opiate, lidocaine, and isoflurane all serve to keep her from facing the horrors of past vivisections. And yet, the whole ordeal must be a nightmare to her. A cumbersome piece of plastic has replaced the skin on the top of her head. She can’t see it, so in the first day after the surgery, whenever she moved too close to the water spout, she bumped up against it with her implant, unable to figure out why something was preventing her from running forward as she intended. She acclimated to it soon enough, and then, after about a week, the recordings began. For the rat, this meant that there was now a thin wire snaking up out of the electronics on her head, tethering her to a computer much like a string connects a child’s hand to a balloon.
Currently, GRat66 has shelter, food, plenty of peanut butter, and not even an inkling of what it’s like to live out in a “real world” full of predators who might eat her at any moment. After the recording, she will have a peaceful few months while I analyze her data. At the end, though, I will need one last thing from GRat66, to verify if the experiment was successful. That thing is her brain, and I will kill her for it.
Grigori Guitchounts is a neuroscientist.