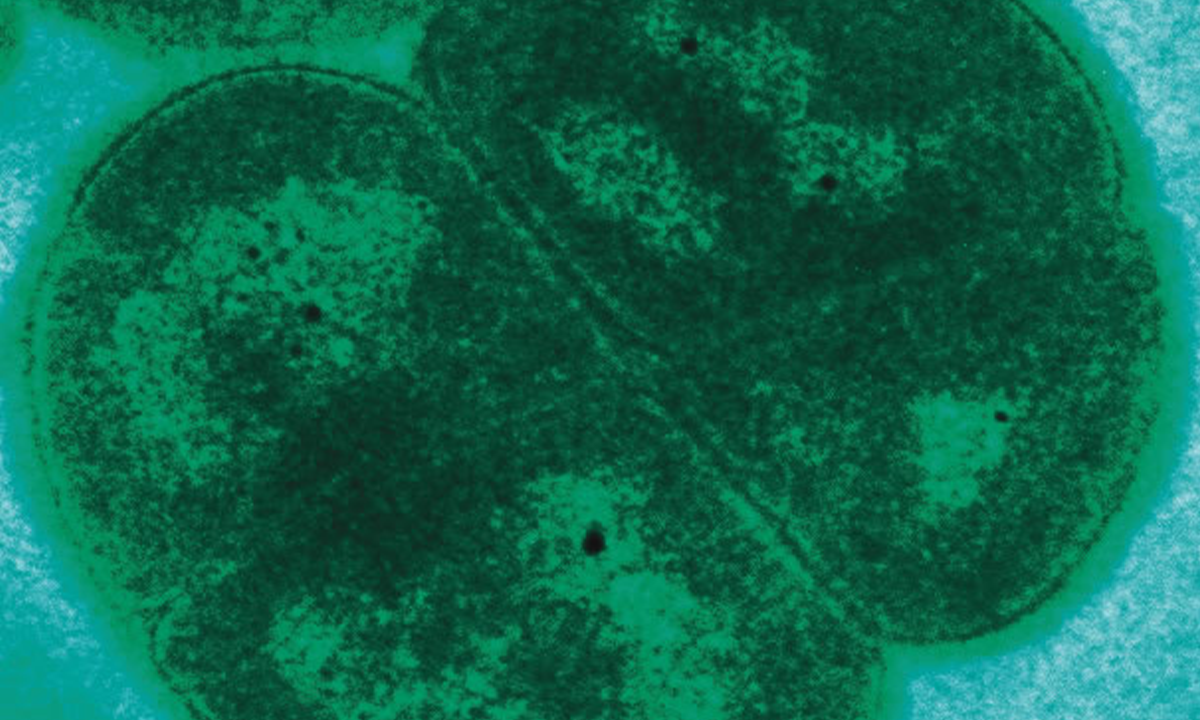
It may have been the slightly dirty glass you drank from at the picnic. Or it may have been the water you accidentally gulped down while swimming in the lake. Or maybe you just didn’t wash your hands enough after using the public bathroom. But somehow, somewhere, you managed to swallow about a dozen hairy, rodlike germs named shigella. They slipped down your esophagus and snuggled next to your intestinal wall. Each then drew out a tiny pincer, punctured your cells, and injected them with a chemical cocktail designed to disrupt the protective outer membrane that normally keeps dangerous invaders out. Confused by the chemicals, your cells opened up and let shigella in, where it quickly set to work, chewing up your insides.
When your diarrhea turns bloody, you rush to the doctor’s office. The doctor starts you on azithromycin, an antibiotic commonly prescribed for intestinal infections like the garden-variety stomach bug. The problem is that the particular shigella you picked up isn’t a garden variety. This shigella is tough. Its ancestors have stood up to a slew of different antibiotics, and while they saw scores of their relatives die, they were the ones that lived. They are very well equipped to combat your antibiotic weaponry.
You take your first dose and the second one, hoping for quick relief. But that’s not what happens. Shigella has already detected the antibiotic molecules, so its genetic defenses are feverishly chugging out a special enzyme that slices azithromycin like a pair of scissors. Three days later, exhausted and worried, you’re back to the doctor. You leave with a prescription for ciprofloxacin or Cipro, a stronger, broad-range antibiotic that should kill everything in its path, including your own, beneficial intestinal microorganisms. It may damage your microbiome, but you have no choice. You leave relieved, sure that the end is in sight.
Four people die every hour from an antibiotic-resistant bacterial infection in the United States.
Two days later, dehydrated, with your fever spiking, your heart racing, and your blood pressure plummeting, you wind up in a hospital, hooked up to an IV. The hospital’s infectious disease specialist starts you on colistin, the antibiotic of last resort, which doctors use only when all else fails because of its long list of side effects, from chest pains to numbness. But you’re heading into kidney failure, so it’s a question of life and death. Either you or the bug has to go.
Unfortunately, this shigella has more genetic tricks up its hairy sleeve, or rather inside its DNA. It finds a way to dodge colistin, too. As it multiplies inside you, it shifts the structure of its outer cell membrane ever so slightly—just enough to make it impervious to colistin, because the medicine can no longer cling to the germ and destroy it. It’s as if the bug—a superbug, really—wraps itself in an invisibility cloak. You may feel better while colistin initially kills all the vulnerable shigella, but they’re soon replaced by a population the antibiotic can’t destroy. Your fever spikes again, your kidneys shut down, and your heart follows.
Sadly, you’re far from being an exception. Four people die every hour from an antibiotic-resistant bacterial infection in the United States. That’s why some scientists say that we’ve entered the post-antibiotic era.
It may not have to be this way. There’s an alternative to destroying invasive germs that scientists have known about for over 100 years, and is now finally being tested in the U.S. These are bacteriophages, or phages for short, a special type of virus that preys on bacteria only. With oblong bodies, spidery legs, and sharp, scorpion-like tails, bacteriophages look like miniature rocket ships from outer space. A thousand times smaller than their prey, they pierce bacteria with their tails, sneak in like Trojan horses, and burst the germs open.
Bacteriophages don’t injure any other cells or tissues in our bodies. Instead, these viruses can destroy the harmful bacteria and leave intact the beneficial ones that help us digest food or protect us from infections. They are the healing viruses, unlike COVID-19 or Ebola or countless others that cause deadly diseases. Had you taken a shigella bacteriophage, you likely would have recovered within a few days.
“Phages can be powerful allies in our rapidly escalating superbug fight,” says Alexander “Sandro” Sulakvelidze, a microbiologist and founder of Intralytix, the first American biotech company to get a grant from the National Institute of Allergies and Infectious Diseases to test phage medicines in humans. “Phages have been preying on bacteria for millions of years before humans came along. They are very good at it.”
It may sound bizarre that viruses can heal, cure, and protect against disease, but phages play a vital role in the human virome—a viral counterpart of our microbiome, which safeguards us from pathogens we encounter constantly. “Phages naturally live in and on us—in our nose, throat, skin, and gut, protecting us from harmful bacteria,” Sandro says. That’s why phages are sometimes called living medicines.
Sandro grew up in Tbilisi, Georgia, where phage medicines were sold in little vials at every pharmacy, next to aspirin. People drank phage remedies for stomach bugs. They gargled with them to cure sore throats. Made at Tbilisi’s Institute of Bacteriophage, Microbiology, and Virology, an organization so well hidden behind the Iron Curtain that barely anyone in the West had heard of it, the remedies were part of the everyday first aid tool kit. People kept them in medicine cabinets. They packed some when they went on vacation.
The institute was founded by a luminary and tragic Soviet-era scientist, Giorgi Eliava, who was executed during Stalin’s Great Terror. (The institute was renamed after Eliava in 1988.) It sat on the banks of the Mtkvari River, from where many of the phages were sourced. The research team cultivated the phages to make medicines against the most notorious scourges—shigella, cholera, staph. They loaded phages into bottles and ampoules and shipped them to pharmacies in Georgia and other parts of the Soviet Union.

Patients battling stubborn skin, throat, ear, and gut infections flocked to the institute from across the country. The Eliava doctors took their swabs and stool samples, identified bacterial culprits, and matched them with phages that preyed on those specific strains. It was personalized medicine at its finest—decades before the term was even coined. Sometimes phages worked fast enough that patients could go home after a few days. Stubborn infections took several weeks or even months. Sometimes patients went home with a supply of ampoules. The most obstinate cases sometimes required extra research and trial and error in assembling a personalized phage mix—formulated specifically to match the bacterial strains the person carried.
To the American medical establishment, phages were as foreign as space aliens. The antibiotics reigned supreme because they were easy to make, had a long shelf life, and worked the same way in every patient. Compared to them, phages, which were viral concoctions developed in the U.S.S.R., didn’t sound trustworthy enough. Sandro, who founded Intralytix in 1998, worked long and hard to change this view. When the FDA finally green-lighted the Intralytix Crohn’s trial in 2018— the first such trial in the agency’s history—it marked a shift in mindset.
Sandro explains that as living medicines, phages are very different from antibiotics. Antibiotics are fixed, static molecules that poison bacteria. Typically, they work by breaking bacterial cells or halting their replication. The cells wither or rupture, spilling out their microbial guts and dying. But we have used antibiotics too often. In response, the bugs mutated their genes and learned to produce their own molecules that destroy our antibiotic ones. Some germs produce enzymes that act like molecular scissors, shredding antibiotic molecules before they have a chance to act. Other germs surround themselves in protective cloaks or eject antibiotics from their cells before they take effect. That’s the unfortunate side effect of evolution. We bred our superbugs ourselves.
People drank phage remedies for stomach bugs. They gargled with them to cure sore throats.
We used to think that we could outpace bacterial evolution with our pharmaceutical prowess, but they mutate faster than we can keep up. Phages do the guesswork for us. Unlike the static molecules of antibiotics, phages are biological entities that fend for themselves—attacking, multiplying, and mutating like all other creatures on Earth. Phages have been feeding on bacteria for eons, so they are equipped to keep up with bacterial evolution. Scientists don’t have to invent a new phage if it loses grip on its prey. They just need to slosh phages and bacteria together in a test tube, let them fight each other, and harvest the winners.
Phages have other advantages, too. They are highly specialized in their microbial diet. A particular phage will only kill the specific bacteria it feeds on, leaving others intact. Phages that kill shigella or salmonella will not harm acidophilus or firmicutes—our native gut bacteria, which we need for digestion and nutrient absorption. That’s a big win over antibiotics that carpet-bomb our intestinal tract, wreaking havoc on our microbiome. Unlike taking antibiotics, drinking phages would destroy only the pathogenic bugs while leaving the good ones intact.
Finding phages is also easier than making antibiotics. Phages are abundant in water, soil, and especially in sewage. “They are the most plentiful biological entities in any habitat, but sewage is particularly good for phage-hunting because it’s teeming with various bacteria that becomes phage food,” Sandro says. To date, Intralytix has harvested hundreds of species of phages, most of which were fished out from sewage plants with the goal of eventually turning them into medicinal agents. Sandro’s team still makes such phage-hunting trips, because not every phage will devour every bug. Intralytix specializes in identifying the right phages for the right bacteria.
“Given the near-infinite number of combinations one needs to test, it’s not a task a human can muster in a reasonable amount of time,” Sandro explains to me in the Intralytix lab. “It would take months, if not years. So we don’t use humans anymore. We use robots. Let me introduce you to Neptune.”
A biotech robot the size of a living room with a price tag of $2 million, Neptune stages microbial warfare between bacteria and phages inside a 96-well plate—a set of shallow tubes. Humans set the stage: pipette in the raw sewage; load up the target bacteria; and supply some bouillon to feed the bacteria so they can grow and multiply, becoming phage food. When loaded, Neptune whirs to life, lining up seven 96-well battlefields, doling out precise amounts of bouillon, bugs, and phages. Finally, it whisks the plates into an incubator attached to its back, where phages will mount their attack.
“It has a way of reading how many bacterial cells are floating in the solution,” Sandro explains, as I watch Neptune’s manipulations, mesmerized by its precision and perfection. “It will do these reads every half hour to see whether bacteria are proliferating or decreasing. At first, their population will grow, but then it should drop. That’s how we know we’ve got good phages.”
To isolate phages against specific germs, such as shigella, Neptune will preload the plates with shigella’s favorite meal—a soup of glucose, amino acids, and iron, which it finds inside our gut. Then Neptune drops the same amount of shigella in every well before adding different shigella phages, or different combinations of them, into the wells. This reveals which phage will extinguish the shigella most efficiently. With that information, Neptune can whip up a remedy recipe for any number of bacterial strains. With the aid of an artificial intelligence network, it can extrapolate that information for novel strains and phages to attack them.
Bacteriophage meds are not coming soon to your local pharmacy. But phage therapy in the U.S. has finally turned the corner. The National Institute of Allergy and Infectious Diseases now awards grants for phage research. There are at least 50 phage clinical trials in progress. Phage startups are mushrooming, signaling a milestone change. The superbugs are here, and phages might prove to be our best weapon. ![]()
Adapted from The Living Medicine: How a Lifesaving Cure Was Nearly Lost—and Why It Will Rescue Us When Antibiotics Fail by Lina Zeldovich. Copyright © 2024 by the author and reprinted by permission of St. Martin’s Publishing Group.
Lead image: Kateryna Kon / Shutterstock