“Some part of our being knows this is where we came from. We long to return. And we can. Because the cosmos is also within us. We’re made of star stuff. We are a way for the cosmos to know itself.”
—Carl Sagan, Cosmos
That Carl Sagan quote is among one of his most famous sayings, and for good reason: it expresses a joy, a connection between us and the cosmos that gives his famous television series its title. It’s (dare I say) a fact so romantic that Neil DeGrasse Tyson called it the most astounding piece of knowledge he would share about the universe.
It’s a stirring thought: nearly every atom of our bodies passed through a star, and many were forged by stars as well. That’s also true for the rocks under our feet, the gases filling our lightbulbs, the air we breathe, even labradoodles. Our galaxy contains billions of planets, both wanderers and homebodies, and it’s a near-certainty that the Milky Way isn’t unique in that regard. Each one of those planets, whether life-bearing or not, is made of star stuff. It’s even more amazing when you consider that much of the “normal” stuff of Earthly life—carbon, oxygen, nitrogen, iron, calcium, sulfur, and so forth—comprises about 1% of all atoms in the universe.
We are common; we are rare.
The story of the atoms in our bodies is the story of the universe in a real sense. A proton in the nucleus of an iron atom in a red blood cell may have been born in the Big Bang, passed through several stars, and been flung across the galaxy before ending up at its current place in your anatomy.

Most of the atoms in the cosmos are hydrogen—the simplest atom, whose just a single proton for a nucleus—while most of the rest are helium, the next-smallest atom. Most of those atoms (along with small amounts of lithium and boron) were born in the intense few minutes following the Big Bang. Despite the intense heat and pressure, there simply wasn’t time to create many heavier nuclei. The early universe was chemically simple.. When the unvierse’s first generation of stars coalesced, they were mostly made of single electrons and protons (that is, hydrogen), since that’s what was available. According to our models, however, these stars were huge, so they quickly fused their available hydrogen into helium, and were correspondingly short-lived. Once the hydrogen supply in its core is exhausted, a star undergoes a series of end-life expansions and contractions, with new fusion processes forging carbon, oxygen, and—if the star is sufficiently massive—heavier elements. (Our Sun is smack in the middle of the main part of its life, about 5 billion years from running out of hydrogen fuel.)
For the early, huge stars in the universe, their lives ended abruptly in supernova explosions, which are hot and energetic enough to produce new elements on their own, providing both a destructive and creative environment in one place. Even as they tear the star apart, the explosions create and fling new chemical elements out into space. The first stars may have been born of hydrogen, but they died in a cloud of heavier atoms. These new elements were still not numerous compared to the primordial hydrogen and helium, but even their small numbers were sufficient to begin changing the chemistry of the cosmos.
The chemistry of life also began here. Hydrogen was born in aftermath of the Big Bang, but without the oxygen from stars, there could be no water. Your body is mostly made of water (though how much percentage-wise depends on how you calculate it), and all life as we know it depends on liquid water. The hydrogen in that water passed through a star to join with the star-made oxygen, to become part of the cytoplasm in a skin cell of your epidermis billions of years later.
The second generation of stars formed out of the debris of the first, and not all of these were huge and fast-burning; many are still around today. Those that were massive, however, had similarly short lifetimes, and also died in supernovas, distributing yet more elements out into space. The iron in the hemoglobin in your blood was made in such an explosion.
Supernovas are not the only way dying stars spread the chemical wealth around. The atmospheres of huge dying stars are only tenuously bound by gravity. As they pulsate, they shed huge amounts of gas in the form of stellar wind. (The analog for the Sun is the solar wind, which thanks to our star’s quieter life is a lot calmer.) The cooler environment away from the star allows the atoms to come together to form molecules: methane and carbon dioxide, some of the precursors of life.
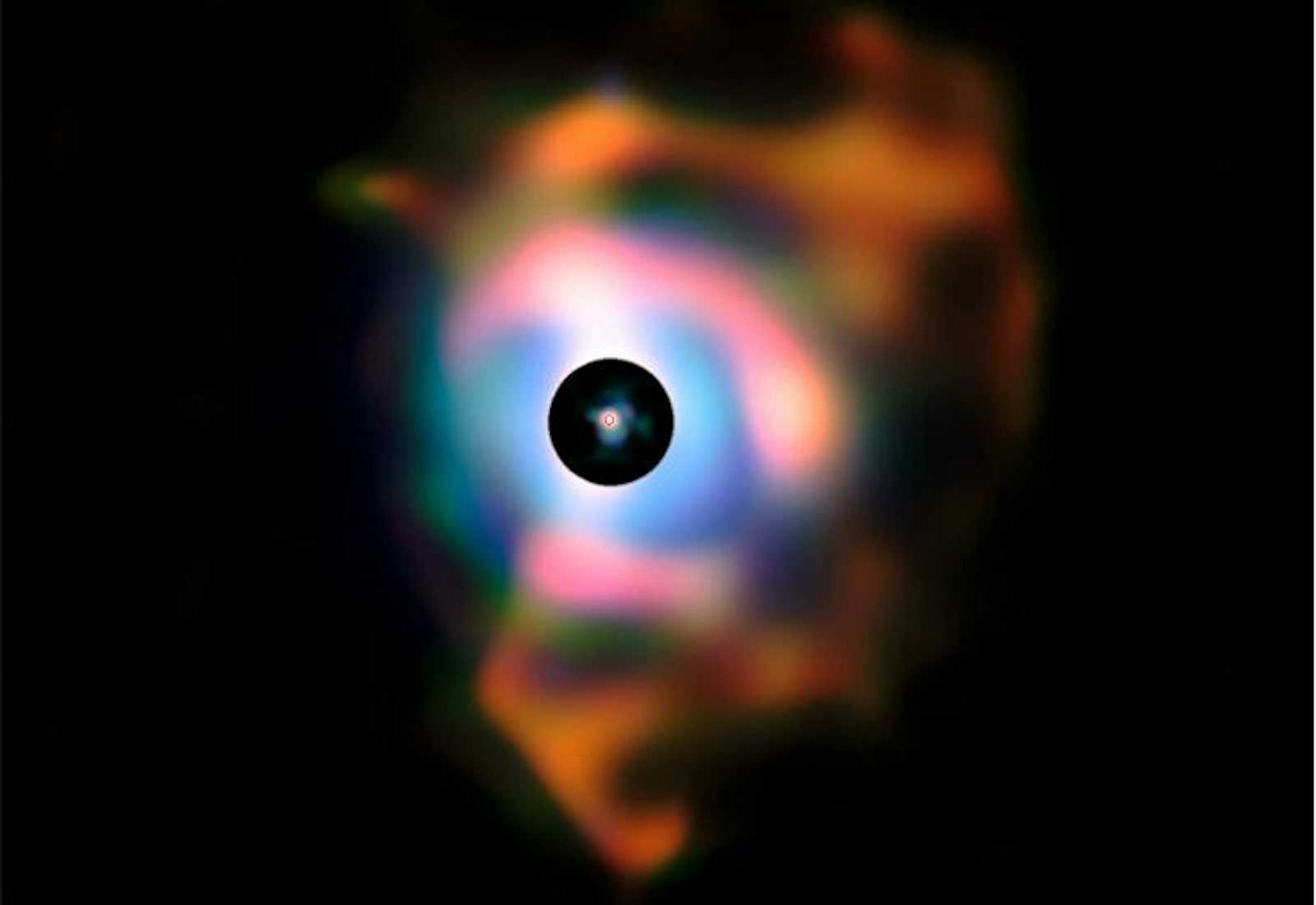
Betelgeuse—the bright red star forming the “shoulder” of the constellation Orion—is one such dying giant. Observations using the Very Large Telescope (VLT) in Chile discovered that Betelgeuse is surrounded by a huge cloud of ejected material, nearly 2 billion kilometers across. The cloud contains a wealth of oxygen-rich molecules, especially silicon dioxide—which on Earth is common beach sand. An atom of silicon in your own aorta was swept into space by stellar winds.
Outside of stars’ cores are their envelopes, which are too cold to fuse atoms, yet act as a different kinds of nuclear reactors. Neutrons from the core bombard atoms in the star’s outer layers, creating heavier elements that are carried away by stellar wind. Even the rare element technetium, which is unstable and not naturally occurring on Earth (its name even means “artificial”), is produced in the envelopes of giant stars. Some of the calcium in your teeth and the milk you give to nurse your baby was also forged this way.
Even lower-mass stars, which don’t die explosively, shed their outer layers gently in the form of planetary nebulas, seeding space with elements and organic molecules, including amino acids, the building blocks of proteins found in you and every other organism. Other kinds of stellar death—the explosion of a white dwarf and the collision of pulsars—could also be responsible for some atoms.
The Sun, with its planets and beings who sit around thinking about this stuff, is neither a first- nor second-generation star. Our solar system formed from a nebula that was itself the graveyard of at least one star, which contained enough iron and nickel to make Earth, the Moon, and the other rocky worlds. In fact, researchers have identified two grains of silicon dioxide in meteorites—almost unimaginably small samples—containing isotopes of oxygen that aren’t known to form from stellar atmospheres, but match those produced in supernovas.
Whatever the specific source, between fusion and other nuclear processes in dying stars, with winds and explosions to disperse their products, stars provide nearly every element found on Earth and the means to bring them into space. The tale of these atoms and their journey from the beginning of time to our anatomy is our backstory. Even better, it’s a story we share with labradoodles and meteorites, stones and running brooks. I would not change it a bit.
Matthew Francis is a physicist, science writer, public speaker, educator, and frequent wearer of jaunty hats. He’s currently writing a book on cosmology, with the working title, Back Roads, Dark Skies: A Cosmological Journey.