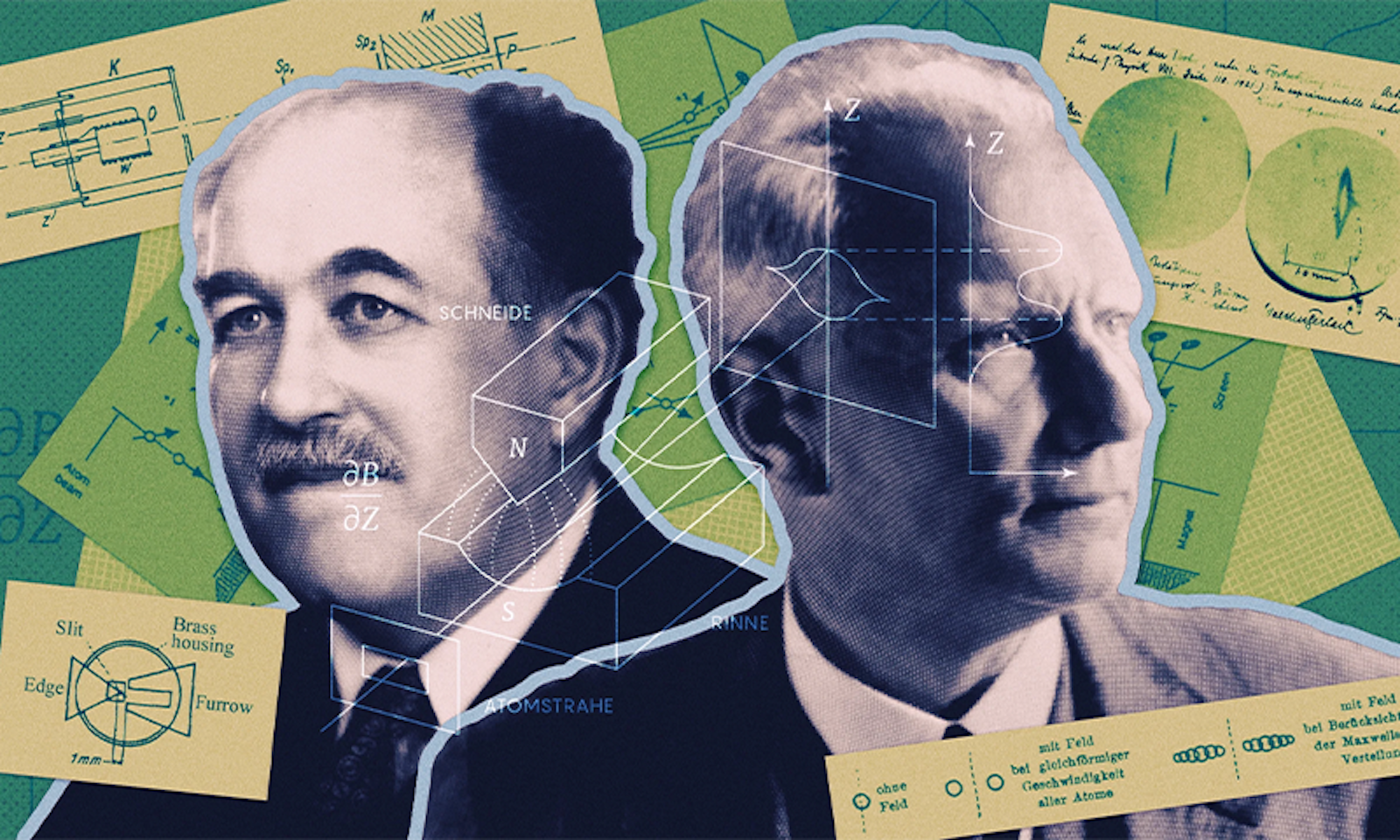
Before Erwin Schrödinger’s cat was simultaneously dead and alive, and before point-like electrons washed like waves through thin slits, a somewhat lesser-known experiment lifted the veil on the bewildering beauty of the quantum world. In 1922, the German physicists Otto Stern and Walther Gerlach demonstrated that the behavior of atoms was governed by rules that defied expectations—an observation that cemented the still-budding theory of quantum mechanics.
“The Stern-Gerlach experiment is an icon—it’s an epochal experiment,” said Bretislav Friedrich, a physicist and historian at the Fritz Haber Institute in Germany who recently published a review and edited a book on the subject. “It was indeed one of the most important experiments in physics of all time.”
The experiment’s interpretation also launched decades of argument. In recent years, physicists based in Israel have finally been able to fashion an experiment with the requisite sensitivity to clarify exactly how we should understand the fundamental quantum processes at work. With that accomplishment, they crafted a new technique for exploring the boundaries of the quantum world. The team will now attempt to modify Stern and Gerlach’s century-old setup to probe the nature of gravity—and perhaps build a bridge between the two pillars of modern physics.
Vaporizing Silver
In 1921, the notion that the conventional laws of physics differed at the smallest scales was still quite contentious. The new reigning theory of the atom, proposed by Niels Bohr, lived at the crux of the argument. His theory featured a nucleus surrounded by electrons in fixed orbits—particles that could whirl only at certain distances from the nucleus, with certain energies, and at certain angles within a magnetic field. The constraints in Bohr’s proposal were so rigid and seemingly arbitrary that Stern pledged to quit physics should the model prove correct.
Stern’s dreams of impugning quantum theory backfired; instead, he won a Nobel Prize.
Stern conceived of an experiment that could invalidate Bohr’s theory. He wanted to test whether electrons in a magnetic field could be oriented any which way, or only in discrete directions as Bohr had proposed.
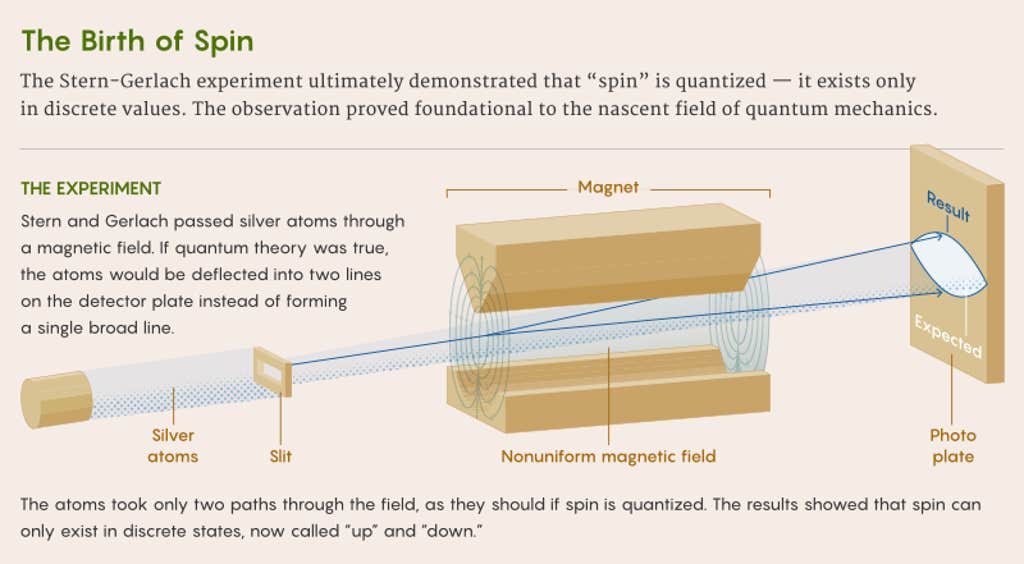
Stern planned to vaporize a sample of silver and concentrate it into a beam of atoms. He’d then shoot that beam through a nonuniform magnetic field and collect the atoms on a glass plate. Because individual silver atoms are like small magnets, the magnetic field would deflect them at different angles depending on their orientations. If their outermost electrons could be oriented willy-nilly, as classical theory predicted, the deflected atoms would be expected to form a single broad smear along the detector plate.
But if Bohr was correct, and tiny systems like atoms obeyed strange quantum rules, the silver atoms could take only two paths through the field, and the plate would show two discrete lines.
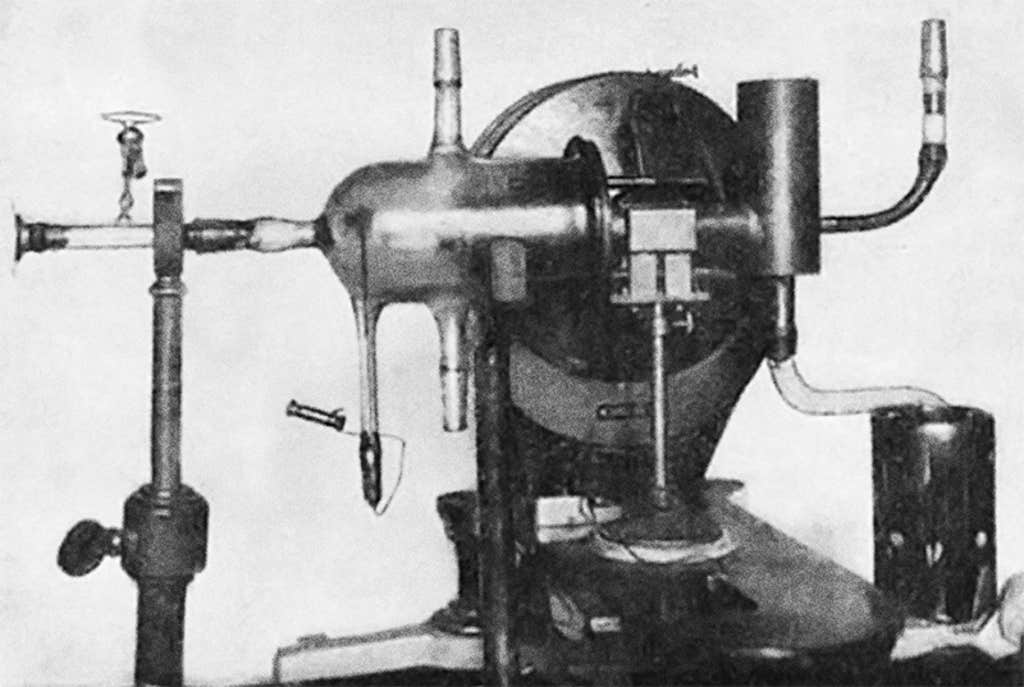
Stern’s idea was simple enough in theory. But in practice, building the experiment—which he left to Gerlach—amounted to what Gerlach’s graduate student Wilhelm Schütz later described as “Sisyphus-like labor.” To vaporize the silver, the scientists needed to heat it to more than 1,000 degrees Celsius without melting any of the seals on the glass vacuum chamber, whose pumps also regularly shattered. The experiment’s funds ran dry as Germany’s postwar inflation soared. Albert Einstein and the banker Henry Goldman eventually bailed out the team with their donations.
Once the experiment was running, producing any legible result was still a challenge. The collector plate was only a fraction of the size of a nail head, so reading the patterns in the silver deposit required a microscope. Perhaps apocryphally, the scientists inadvertently helped themselves out with questionable laboratory etiquette: The silver deposit would have been invisible if it weren’t for the smoke trickling in from their cigars, which—because of their low salaries—were inexpensive and rich in sulfur that helped the silver develop into visible jet-black silver sulfide. (In 2003, Friedrich and a colleague reenacted this episode and confirmed that the silver signal appeared only in the presence of cheap cigar smoke.)
The Spin of Silver
After many months of troubleshooting, Gerlach spent the entire night of February 7, 1922, shooting silver at the detector. The next morning, he and colleagues developed the plate and struck gold: a silver deposit neatly split in two, like a kiss from the quantum realm. Gerlach documented the result in a microphotograph and shipped it as a postcard to Bohr, along with the message: “We congratulate you on the confirmation of your theory.”
The finding shook the physics community. Albert Einstein called it “the most interesting achievement at this point” and nominated the team for a Nobel Prize. Isidor Rabi said the experiment “convinced me once and for all that … quantum phenomena required a completely new orientation.” Stern’s dreams of impugning quantum theory had obviously backfired, though he did not hold to his promise of quitting physics; instead, he won a Nobel Prize in 1943 for a subsequent discovery. “I still have objections to the … beauty of quantum mechanics,” Stern said, “but she is correct.”
Today, physicists recognize that Stern and Gerlach were right in interpreting their experiment as a corroboration of the still-nascent quantum theory. But they were right for the wrong reason. The scientists assumed that a silver atom’s split trajectory is defined by the orbit of its outermost electron, which is fixed at certain angles. In reality, the splitting is due to the quantization of the electron’s internal angular momentum—a quantity known as spin, which wouldn’t be discovered for a few more years. Serendipitously, the interpretation worked out because the researchers were saved by what Friedrich calls a “strange coincidence, this conspiracy of nature”: Two yet-unknown properties of the electron—its spin and its anomalous magnetic moment—happened to cancel out.
Cracking Eggs
The textbook explanation of the Stern-Gerlach experiment holds that as the silver atom travels, the electron isn’t spin-up or spin-down. It’s in a quantum mixture or “superposition” of those states. The atom takes both paths simultaneously. Only upon smashing into the detector is its state measured, its path fixed.
But starting in the 1930s, many prominent theorists opted for an interpretation that required less quantum magic. The argument held that the magnetic field effectively measures each electron and defines its spin. The idea that each atom takes both paths at once is absurd and unnecessary, these critics argued.
In theory, these two hypotheses could be tested. If each atom really did traverse the magnetic field with two personas, then it should be possible—theoretically—to recombine those ghostly identities. Doing so would generate a particular interference pattern on a detector when they realigned—an indication that the atom indeed navigated both routes.
The grand challenge is that, to preserve superposition and generate that final interference signal, the personas must be split so smoothly and quickly that the two separated entities have wholly indistinguishable histories, no knowledge of the other, and no way of telling which path they took. In the 1980s, multiple theorists determined that splitting and recombining the electron’s identities with such perfection would be as unfeasible as reconstructing Humpty Dumpty after his great fall from the wall.

In 2019, however, a team of physicists led by Ron Folman at Ben-Gurion University of the Negev glued those eggshells back together. The researchers started by reproducing the Stern-Gerlach experiment, although not with silver, but with a supercooled quantum conglomeration of 10,000 rubidium atoms, which they trapped and manipulated on a fingernail-size chip. They put the rubidium electrons’ spins in a superposition of up and down, then applied various magnetic pulses to precisely separate and recombine each atom, all in a few millionths of a second. And they saw the exact interference pattern first predicted in 1927, thus completing the Stern-Gerlach loop.
“They were able to put Humpty Dumpty back together again,” Friedrich said. “It’s beautiful science, and it’s been a huge challenge, but they’ve been able to meet it.”
Growing Diamonds
Aside from helping to verify the “quantumness” of Stern and Gerlach’s experiment, Folman’s work offers a new way to probe the limits of the quantum regime. Today, scientists still aren’t sure just how big objects can be while still adhering to quantum commandments, especially when they’re large enough for gravity to intervene. In the 1960s, physicists suggested that a full-loop Stern-Gerlach experiment would create a super-sensitive interferometer that could help test that quantum-classical boundary. And in 2017, physicists expanded that idea and suggested shooting tiny diamonds through two neighboring Stern-Gerlach devices to see if they gravitationally interacted.
Folman’s group is now working toward that challenge. In 2021, they outlined a way to beef up their single atom-chip interferometer for use with macroscopic objects, such as diamonds comprising a few million atoms. Since then, they’ve shown in a series of papers how splitting larger and larger masses will again be Sisyphean, but not impossible, and could help solve a slew of quantum gravity mysteries.
“The Stern-Gerlach experiment is very far from completing its historical role,” Folman said. “There’s still much that it’s going to give us.”
This article was originally published on the Quanta Abstractions blog.
Lead image: Otto Stern (left) and Walther Gerlach set out to challenge quantum mechanics. Instead, their experiment proved foundational to the newly born field. Credit: Kristina Armitage/Quanta Magazine; source: Deutsches Museum; Niels Bohr Archive.