When Copernicus told us more than 400 years ago that the Earth was not at the center of the universe, he could hardly have imagined that it would lead this far. At the hands of astronomy and cosmology, we seem to have been reduced to near nothingness, specks within slivers of time and space, inside specks that are themselves entire universes. But how should we interpret this fact? Does this ultimate extension of the Copernican narrative seal our infinitely mediocre fate? The question is more complex than it initially seems.
It’s easy to feel unimportant
It starts in our own galactic backyard, around the star Alpha Centauri B. Last year, we discovered that our neighbor harbors at least one world [1].1 It’s not the kind of place we’d call home, mind you. Its year is only three days long. Its surface temperature may be more than a thousand degrees Celsius—enough to melt silicate rock. You would be immersed in scorching mineral vapor on arrival, flash cooking your lungs—that is, before you exploded in an evaporative puff.
But it is Earth-sized, and it is only four light years away. There could be other, more temperate planets in this system. These worlds would, in turn, be merely the nearest motes among our cosmic neighbors. Our Milky Way galaxy has more than 200 billion stars, and is one of a few hundred billion galaxies [2] in the observable universe. And we have learned in the last few years that a flotsam and jetsam of worlds forms around this dizzying starscape with a shocking degree of efficiency. (See Planet Detection)
In 1995, we detected the first planets outside of our solar system (exoplanets) around sun-like stars. Since then, the count of known exoplanets has rocketed to a few hundred, with 2,700 robust candidates and as many as 18,000 stars in need of further study. Some star systems are positively bursting with worlds, packed into orbits that hover at the hairy edge of gravitational chaos. In fact there may easily be many more planets than there are stars in the universe—which means hundreds of sextillions of planets, small and big, hot and cold, and, probably, some vaguely like our own Earth. Indeed, recent analyses [3] suggest that at least 15 percent of all stars may have Earth-sized planets orbiting within the zone where liquid water might exist on their surfaces.
Life on these planets is appearing increasingly likely, too. We’ve learned in recent years that the cosmos is filled with the very same building blocks that life on Earth uses. Look outwards and you’ll find that more than 70 percent of the molecules drifting in interstellar space contain carbon atoms, which, with their spare valence electrons, are fantastically good at bonding with other elements.
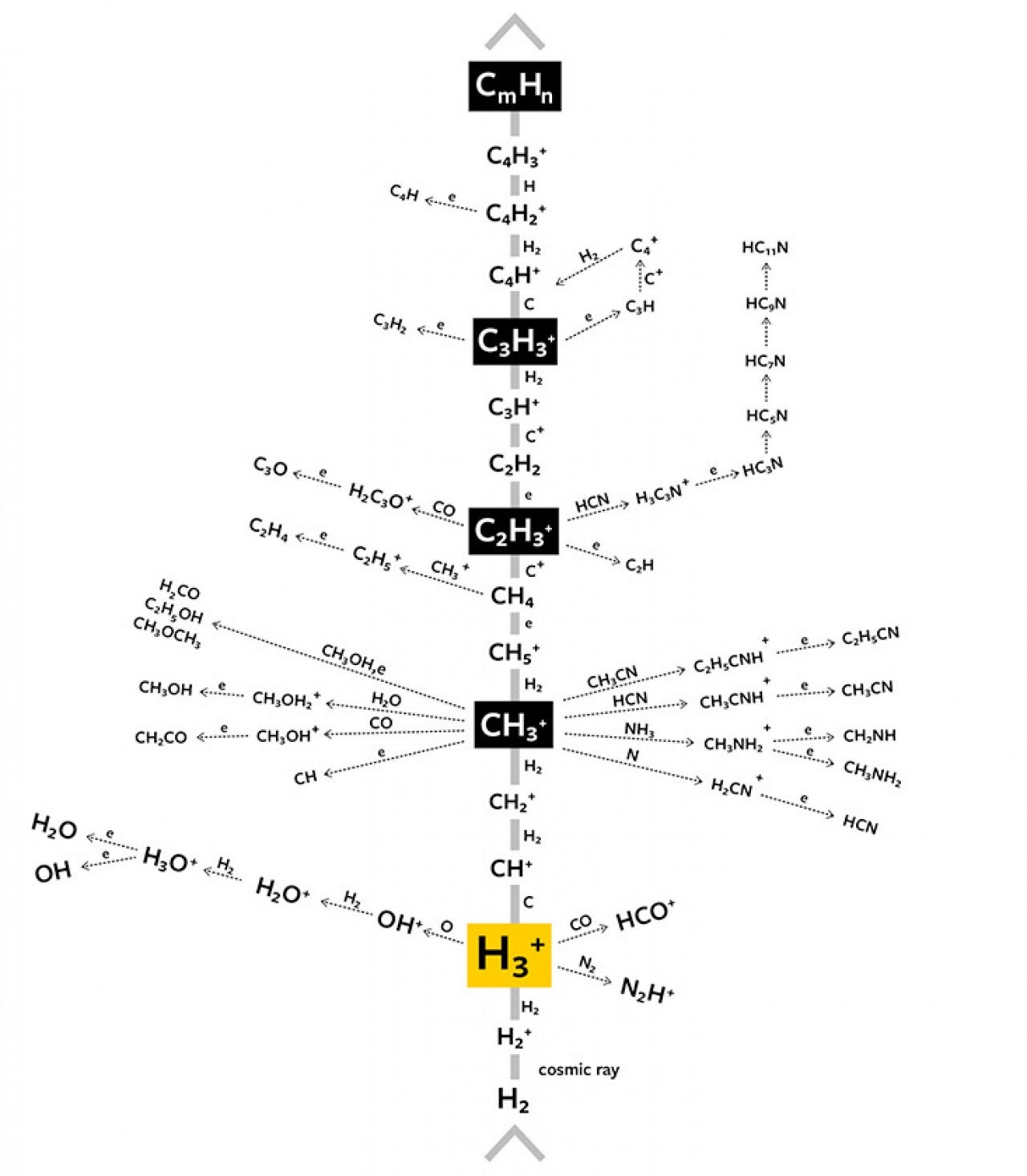
Outer space, it turns out, is a vast slow cooker. The densest interstellar gas may contain 10,000 hydrogen atoms in a cubic centimeter, and three or four carbon atoms. This is only a thousand trillionth of the density of the air we breathe. An atom in deep space may travel 100,000 kilometers before bumping into a partner. But space has its own secret sauce. A long underappreciated bevy of three protons and two electrons called protonated molecular hydrogen [4], or H3+, catalyzes a great network of reactions as elements collide, or stick to the surface of star-produced microscopic silicate and carbon dust grains. (See Interstellar Reactions.) Over many millions of years, compounds as complex as polycyclic aromatic hydrocarbons are manufactured, with dozens of carbon atoms in arrays of benzene rings. Other structures are precursors to amino acids, the stuff you and I are made of. Here on Earth, we can read the chemical record of this activity in fallen meteorites containing dozens of amino acid species. If they are falling on us, they are probably falling on other planets, conceivably forging the start of countless alien biochemistries in a kind of starter mix for life. And my own speculation is that there are many, many different ways to end up with complex organisms, that the steps that happened to allow us to be here on Earth do not need to be copied verbatim elsewhere.
Outer space, it turns out, is a vast slow cooker
Faced with these observations it seems that one would need to invoke some extraordinarily contrived circumstances to argue that the fact and nature of life on Earth is at all special.
Then there is the final frontier—no, not the one from Star Trek, but something far greater than that. Many cosmologists are now convinced that our universe is merely one of a vast array of universe ‘things,’ each with its own set of physical laws, mostly separated by space and time. Some of these ideas spring naturally from the physics driving the exponential, inflationary expansion of the very early universe and the seemingly inherent instability of ‘nothing’ itself. While only speculation today, these ideas may soon be testable. Subtle signatures of our cosmos ‘bumping’ into others could exist in the pervasive cosmic radiation background, or in the motion of matter across vast scales, both amenable to detection by the next generation of astronomical experiments.
Even in the seemingly unlikely case that there is absolutely no other life in the dizzying expanse of our own universe, it is far less likely for there to be none among an indefinite stretch of universes, each with their own physics, their own untold planets, and their own cosmic chemistries.
We are here. Therefore they are there?
Phew. We are in a big, big place. It can be exhausting to think about. And we are a long way from being the center and purpose of creation as imagined by the average scholar just 600 years ago. But what does this vastness really mean for us? How should we react? If we are the only life in these many universes, we are, in some ways, more special—more significant. And there’s the rub. Our reaction depends on whether or not other life is actually out there.
Our quantitative reasoning about the occurrence of life beyond Earth owes a debt to a previously obscure 18th century British mathematician and non-conformist theologian called Thomas Bayes [5]. Bayesian statistics are all about confidence. To illustrate this, imagine, if you will, a rather inexperienced but mathematical gardener [6].2 She has a plot of land and a large bag full of these mysterious things called ‘seeds.’ On a whim the gardener buries one of these seeds in the soil, waters it, and waits.
At this stage she has no idea what will happen next. A couple of days pass and, lo and behold, a shoot appears! But what does this mean? Will seeds always produce shoots? This is a promising possibility and so the gardener formulates a measure of her confidence that this will happen again. Her hypothesis is that there is equal probability of a seed sprouting or not sprouting—the odds are 1-to-1, or 50 percent, for either outcome. This assumption, called a “uniform prior,” is clearly not true—but it is the most logical hypothesis. The gardener knows only that her experiment has two possible outcomes: a sprout, and no sprout. Since there is too little data to bias her one way or the other, she gives equal odds to each outcome.
She goes ahead and plants another seed in the ground, waits a couple of days, and another shoot appears. She has now seen two seeds sprout, and so modifies her confidence to give 2-to-1 odds for planted seeds to sprout (66.6 percent, or two out of three possible outcomes). A third success and this increases to 3-to-1 (75 percent) and so on. With every new sprouting the gardener becomes more and more confident, but never absolutely sure, that planted seeds will grow. Even by the time she’s seen the hundredth shoot appear, she is only about 99 percent confident that this will happen again.
The question of life’s abundance in the universe is ripe for this kind of Bayesian analysis. And in 2011 two Princeton scientists, David Spiegel and Edwin Turner [7], did just that. Their approach was much more sophisticated than the gardener’s, but it shared a key characteristic: they were working with very little data. In fact, the totality of their data can be summarized (a bit glibly) by saying that microbial life ‘sprouted’ quickly here on Earth (an event termed abiogenesis), and some 3.8 billion years later, complex-celled, intelligent life emerged to make this very observation. What do these two facts tell us about the likelihood of life elsewhere in the cosmos?
The answer is, not much. At its core, Bayesian analysis weighs how much our conclusions stem from our prior assumptions and how much comes from our actual data. In this case it reveals that our conclusions about life in the universe are acutely dependent on what we assume—what our prior theory is for the probability of life arising on planets, and to a lesser degree on the specific outcome on Earth. A simple, optimistic model assuming that the frequency of abiogenesis is a constant, or perhaps decreases with planetary age, predicts an abundance of cosmic life when calibrated to abiogenesis here on Earth. But the model admits abiogenesis rates as low as once per 10 billion or even 100 billion years, implying we could also be the first life in the cosmos. Change the model slightly, and all bets are off. There is simply too little data to swing our confidence in either direction.
Furthermore, Spiegel and Turner show that this analysis hinges on the simple fact that, because we are here to ask the question, we must be on a planet that supports the eventual emergence of intelligent life. Unfortunately, as a result, our own existence tells us very little indeed about the probability of life off of Earth: it is, in some sense, an echo of the question itself.
One would need to invoke some extraordinarily contrived circumstances to argue that the fact and nature of life on Earth is at all special.
What their investigation does vividly demonstrate is how enormously our understanding changes if we find just one other example of life in the universe. The probable rate of abiogenesis on a given planet would rise by a factor of 10 or more, to more than once every billion years. The universe is only 13.8 billion years old, so this makes a difference! Suddenly, our galaxy is full of life.
Are we a standard example of carbonaceous life in a universe teeming with it? Simply put, we don’t know. But there is something else I can tell you: science has shown us how life, any life, is in some ways far from mediocre.
At one with the universe
There is something very old, deep, and, yes, significant that might challenge the notion of our mediocrity.
It starts because you’re made of the cumulative stuff of the cosmos. A little more than 3.8 billion years ago some part of you came crashing to Earth. It might have been a handful of your carbon, oxygen, or nitrogen atoms, or some of the many hydrogen nuclei that now exist in your molecules. Primordial things are these, the remains of a hot Big Bang that took place 10 billion years earlier. Pieces of the one-in-a-billion tailings of a universe filled with annihilating matter and antimatter.
Your heavier elements passed through the digestive system of other stars. Cooked up by nuclear fusion in 10 million degree stellar cores. Hidden from sight under seething cloaks of plasma that could be millions of miles deep, these tiny clusters of matter were eventually dispersed to interstellar space in supernovae explosions that could momentarily outshine an entire galaxy. Cooling in the chill of space, they inhabited nebular clouds, eventually once again succumbing to gravity’s relentless embrace to fall inwards to the tumult of a youthful swirl of matter surrounding a growing baby star. Going through this process just once is enough to make a smattering of heavier elements if the star is sufficiently massive, but it takes multiple stellar generations to enrich the universe enough to build worlds like ours, and us. We are well down the family tree.
Back at the birth of our planet the elements arrived first in great embryonic collisions and later in a tapering, diminishing rain of metal, rock, and ice [8].3 In the billions of years that followed, these atoms took many different paths. Some were carried into oceans and atmospheres, others sequestered into the cooling minerals of a floating planetary crust. Trillions upon trillions of microscopic organisms have processed many of these atoms across the eons—perhaps carrying them around for a while, or simply plucking them up for the briefest instant of chemistry before discarding them.
Some atoms were incorporated into other living matter: insects, plants, animals—a wild array of trajectories and histories. Until now, today, when they’ve been passed on from your mother, her food, your food, the air you breath, and the water you drink, to become a piece of your very corpus.
But, if a handful of fundamental physical constants and initial characteristics of the universe were slightly different, these pathways to galaxies, stars, heavy elements, the ubiquitous carbon molecules, and life itself, would be subverted. For example, the fine structure constant determines the size and behavior of atoms. If it were slightly bigger, or slightly smaller, objects like planets might not form, nor would chemistry operate the way it does. If the strength of gravity were a little greater all stars would be blue giants, and planets like Earth could not exist. If it were a little smaller, all stars would be red dwarfs and there would be few, if any, heavy elements.
There are other apparent coincidences. The manufacture of carbon in the bellies of stars relies on the existence of a specific, resonant, excited energy state of carbon-12 nuclei, the unusual stability of beryllium, and the nature of oxygen-16 nuclei. Without all these ducks lined up in a row, there would be no carbon. Without carbon there would be no life. There is also the remarkable coincidence of us being able to discover all of this. (See Middle Ground).
Such observations have led to the Anthropic Principle [9]—the idea that our very presence is somehow relevant to the cosmos. (In truth it need not be our presence, since the presence of any life does the trick. So the ‘anthropic’ is a bit selfish and the principle slightly misnamed). This principle has different variants. A so-called ‘weak’ version is the most straightforward: it says that the apparent fine-tuning for life is a selection bias, since if it weren’t this way we simply wouldn’t be here to examine it. The weak Anthropic Principle was an early argument for the existence of multiple universes, which neatly avoids us having to explain why this universe ended up this way and connects with multiverse ideas emerging from fundamental physics. We simply exist in a universe suited for life. The strong Anthropic Principle goes further still, and argues that a viable universe is somehow compelled to produce life like us—it could not be any other way.
There is even a body of thought that supposes that life is the ultimate end or expression of our universe, the apotheosis of complexity in a vast space that can seem curiously suited for us to exist in it. In fact the late, great physicist John Wheeler mused on whether information is at the root of all physics. A consequence might be that consciousness itself, also built from information, is a key component of reality and that ours is a participatory universe in which consciousness affects reality.
In most respects, the connectedness of life to basic features of our universe, and the Anthropic Principle, are the polar opposite of the Copernican worldview, which can be an uncomfortable thing. But what these facts accomplish is to widen the circle of what we consider to be life “like us.” Life beyond Earth may very well also be carbon-based, initiated by similar combinations of monomers into complex information-carrying compounds, evolving through similar processes of selection, enabled by the same cosmic coincidences. In other words, cut from the same fabric.
Indeed, there is a nagging doubt that any life we find will be truly independent of us. In our own solar system, asteroids have battered planets throughout the past 4.5 billion years, with each collision potentially launching microbe-harboring surface material into a variety of ‘conveyor-belt’ orbital pathways [10]. Pieces of Mars eventually end up on Earth. Pieces of Earth end up on Mars, and even on the distant moons of the giant planets. Nature may have already created an intermixed diaspora of life in our solar system. And it is also possible that life further afield is connected to our own, just through a more ancient bridge.
So if we find life off Earth, the Bayesians will have their work cut out for them. As for our sense of our own significance, we could argue that it would strengthen, by confirming the entrenched nature of life in our universe. On the other hand, we tend to take things personally, and a universe full of life might seem a lot smaller.

Unique vs. significant
We are wrestling with two distinct questions: our uniqueness, and our significance. Uniqueness is easier to approach. For the astrobiologist, uniqueness can be defined as the probability that Earth, and life on Earth, is different from anywhere else. Primatologists, on the other hand, may define uniqueness as the collection of characteristics that distinguish us from our evolutionary cousins. For computer scientists, uniqueness can be defined in terms of the specific way in which we process and store information. In each case, while the answer may not be simple, progress is being made.
In my own field, astronomers and exoplanetologists are actually beginning to construct quantitative measurements of our uniqueness, by evaluating the degree to which alien systems and worlds may or may not be suitable for life. Within 10 years, the James Webb Space Telescope may tell us if nearby terrestrial-sized planets exhibit the chemical signatures of a biosphere. Sniffing their atmospheres through the spectra of filtered starlight. And new, giant, telescopes that are starting to move from blueprints to reality will help do the same.
Significance is trickier, and also, perhaps, a question that astronomy gives a unique perspective on. Certainly the frequency with which any kind of life occurs in our galaxy and in the universe as a whole is relevant. We are in the process of refining this question, asking what detailed properties correspond to what abundance of life and not just how little the universe need be different to prevent all life.
But will this be enough? Like it or not, significance is an emotionally charged question for us humans, and it can mean many different things. It can be about whether our home, our planet, our universe matters. It can also be about whether we matter, whether we’re at all important in this vast cathedral of a cosmos. Or in other words, whether nature gives a damn. It doesn’t seem science can help much with that, at least not yet, and one suspects its answers are unlikely to comfort us. Science has told us, though, that we belong to the universe in interesting and deep ways—that we should think carefully about the Copernican narrative of ever-diminishing importance.
So has science sealed our fate? The answer is a resounding no. Whatever science tells us (and often because of what it tells us), we possess the capacity to take matters into our own hands, to make ourselves significant. Our solar system and the star systems beyond represent a huge terrain for exploration and habitation. Although there are enormous hurdles to traveling to our sister worlds, much less through interstellar space itself, there is nothing obviously impossible about it.
Even if we content ourselves with just sending robotic avatars out into the universe, by doing so we have the opportunity to alter the fundamental balance of the significance equation. By extending our presence we may not only find a way to extend our species’ longeity, but also to modify the most fundamental precepts of the questions we have always asked about our place in the cosmos. We can become the significance that we are looking for.
Caleb Scharf is an astrophysicist and the Director of Astrobiology at Columbia University in New York. His popular science books include Gravity’s Engines: How Bubble-Blowing Black Holes Rule Galaxies, Stars, and Life in the Cosmos, and The Copernicus Complex: A Quest for Our Cosmic (In) Significance (forthcoming April 2014).
References
1. Dumusque, X., et al. An Earth-mass planet orbiting Alpha Centauri B. Nature 491, 207 (2012).
2. Beckwith, S., et al. The Hubble Ultra Deep Field. The Astronomical Journal 132, 1729. http://arxiv.org/abs/astroph/0607632 (2006).
3. Dressing, C. & Charbonneau, D. The Occurrence Rate of Small Planets around Small Stars. The Astrophysical Journal at http://arxiv.org/abs/1302.1647 (2013).
4. McCall, B. & Oka, B. H3+ – an ion with many talents. Science 287, 1941- 1942 (2000).
5. Bayes, T. & Price, R. An Essay towards solving a Problem in the Doctrine of Chance. Philosophical Transactions of the Royal Society of London 53, 370–418. http://www.stat.ucla.edu/history/ (1763).
6. Spiegel, D. & Turner, E. Bayesian analysis of the astrobiological implications of life’s early emergence on Earth. Proceedings of the National Academy of Sciences 109, 395-400 (2011).
7. Crida, A. Solar System Formation. Reviews in Modern Astronomy 21, http:// arxiv.org/abs/0903.3008
(2009).
8. Carter, B. Large number coincidences and the anthropic principle in cosmology. “Confrontation of cosmological theories with observational data,” Proceedings of the Symposium, Krakow, Poland. Dordrecht, D. Reidel Publishing Co. (A75-21826 08-90) 291- 298 (1974).
9. Mileikowsky, C., et al. Natural transfer of viable microbes in space. “From Mars to Earth and Earth to Mars.” Icarus 145, 391-427 (2000).
10. Krauss, L. & Scherrer, R. The Return of a Static Universe and the End of Cosmology. International Journal of Modern Physics D, 17, 685-690 (2008).



























