You may think of yourself as a highly refined and sophisticated creature—and you are. But you are also full of discarded, rejected, and recycled atomic elements. Don’t worry, though—so is almost everyone and everything else.
Carbon: Your inky nails

Look at one of your fingernails. Carbon makes up half of its mass, and roughly one in eight of those carbon atoms recently emerged from a chimney or a tailpipe. Coal-fired power plants, petroleum-guzzling cars, and kitchen gas stoves release carbon dioxide into the atmosphere. Each of those waste molecules is a carbon atom borne on two atomic wings of oxygen. Fossil-based carbon dioxide molecules that are not soaked up by the oceans or stranded in the upper atmosphere are eventually captured by plants, shorn of their oxygen wings, and woven into botanical sugars and starches. Eventually, some of them end up in bread, sweets, and vegetables, while others help form carbon-rich animal tissues, finding their way into meat and dairy products. Historically, atmospheric carbon dioxide was mainly replenished by volcanoes, forest fires, and biotic respiration. Today, one quarter of atmospheric CO2 is the result of fossil fuel combustion, whether it rose from smokestacks or was displaced from the oceans. (When fossil-fuel CO2 dissolves into ocean water, it displaces already-dissolved carbon dioxide derived from natural sources.) And because all of the carbon in your body derives from ingested organic matter, which in turn obtains it from the atmosphere, your fingernails and the rest of the organic matter in your body are built, in part, from emissions.
Radioactive Carbon-14: Your pearly whites

When you smile, the gleam of your teeth obscures a slight glow from radioactive waste. During the late 1950s and early 1960s, atmospheric testing of thermonuclear weapons scattered so much radioactive carbon-14 into the atmosphere that it contaminated virtually every ecosystem and human. Several thousand unstable radiocarbon atoms explode within and among your cells every second as their unstable nuclei undergo spontaneous radioactive decay. Some are the natural products of cosmic rays that can turn atmospheric nitrogen into carbon-14, while others result from the decay of unstable mineral elements that are found in soil. But many of them represent the echoes of thermonuclear airbursts from the Cold War, finding their way into our water supply and meals. If they happen to disintegrate within your DNA, they can damage your genes. And many of them are bound up in your teeth. Unlike most of the atoms in your body, those embedded in your strong, stable tooth enamel have been with you ever since you ingested them through your umbilical cord and your infant feeding. If you were born during the early 1960s, you have more nuclear waste in your teeth than if you were born later, when soils and oceans had had time to bury radioactive atoms. In fact, forensic scientists use the proportion of bomb carbon in tooth enamel to determine the age of unidentified human remains.
Oxygen: Your leafy breath

The oxygen in your lungs and bloodstream is a highly reactive waste product generated by vegetation and microbes. Trees, herbs, algae, and blue-green bacteria split oxygen atoms out of water molecules during photosynthesis. They use most of the resultant gas for their own purposes, but thankfully some leaks out to sustain you. In fact it makes up about a fifth of the air you breathe. Your cells harness oxygen to release energy from chemical bonds in the food you consume. Oxygen absorbs electrons released by broken food molecules, which attract hydrogen ions, resulting in a molecular waste of your own making: metabolic water, which comprises one tenth of your body fluids. An average adult carries between 8 and 10 pounds of homemade wastewater within them, and one in 10 of your tears are the metabolic by-products of your breathing and eating.
Nitrogen: Your natural curls

The next time you brush your hair, think of the nitrogenous waste that helped create it. All of your proteins, including hair keratin, contain formerly airborne nitrogen atoms. But the nitrogen in air is biologically inert. For nitrogen to become a component of your hair, it has to be converted into a more accessible form. Nitrogen-fixing bacteria is one way that can happen. They live among the roots of beans, peas, and other legumes, consuming atmospheric nitrogen and releasing it as ammonia, a kind of microbial manure that fertilizes soil in which plants grow. When you eat a plant, you consume formerly atmospheric nitrogen. Every flash of lightning and every automotive spark plug emits a puff of nitrogen oxide, which can dissolve into raindrops and fall to earth as a form of fertilizer, again finding its way into food webs through plants. But most of the nitrogen in modern foods comes from urea and ammonium nitrate fertilizers artificially fixed by industrial processes. In ages past, the nitrogen in human hair came mainly from bacterial waste and lightning. But today, unless you eat a strictly organic diet, you run your hairbrush through nitrogenous frameworks that are mostly of human origin.
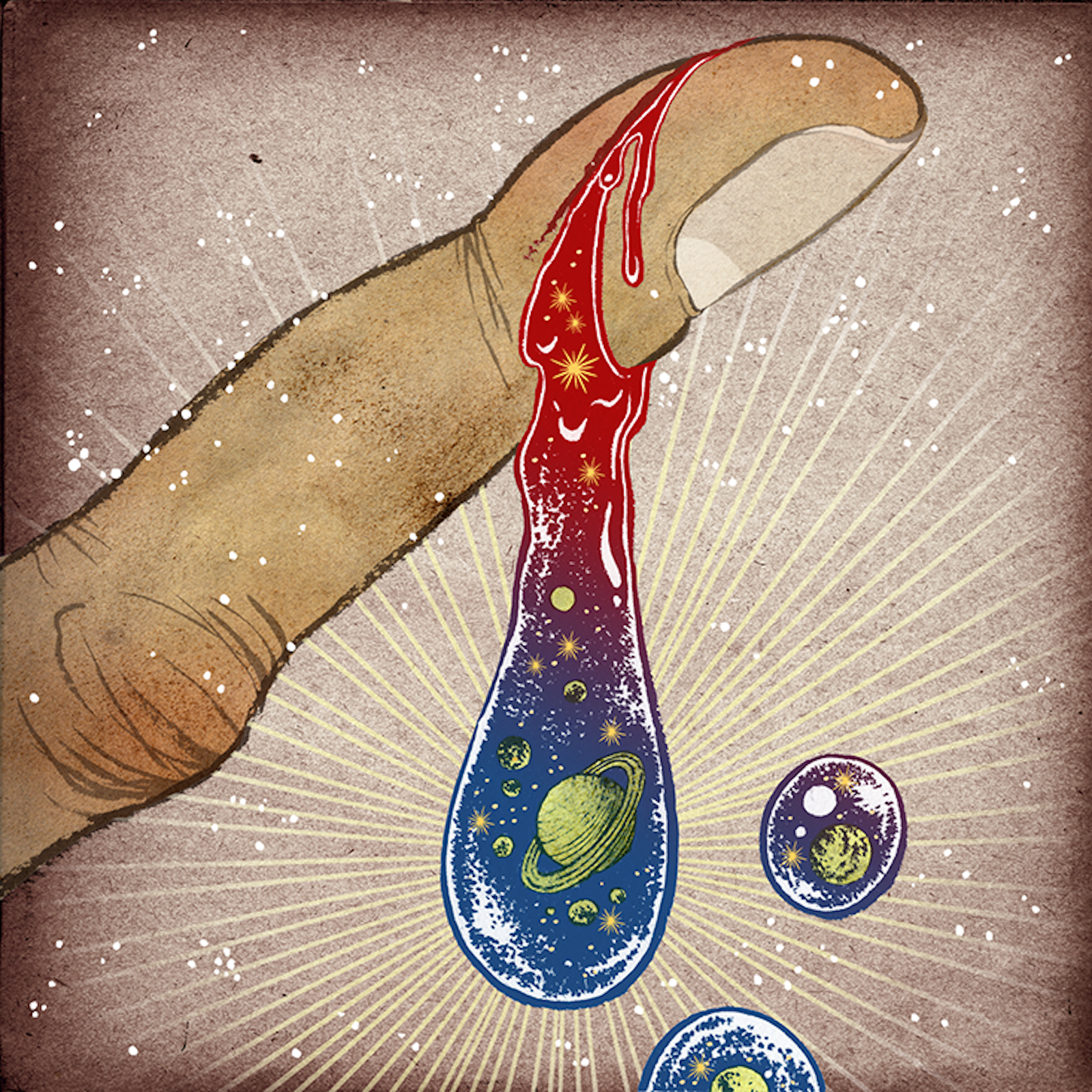
Iron: Your ancient blood
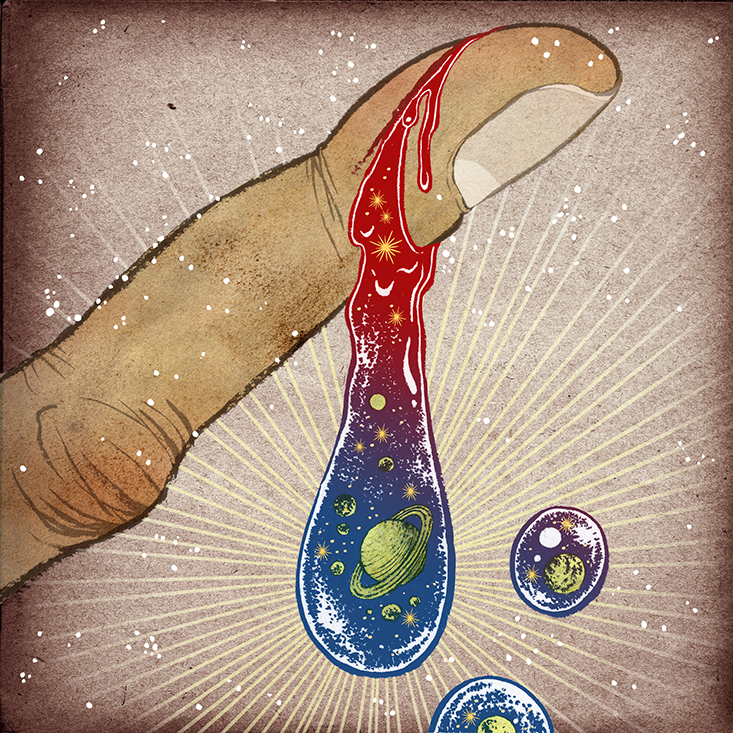
When you cut yourself, the wreckage of stars spills out. Every atom of iron in your blood, which helps your heart shuttle oxygen from your lungs to your cells, once helped destroy a massive star. The fierce nuclear fusion reactions that set stars ablaze create the atomic elements of life. As the star ages, it fuses progressively larger elements, such as silicon, sulfur, and calcium. Eventually, iron atoms are fused. The problem is that iron fusion consumes as much energy as it produces, so it weakens the star. If the star is big enough, it will collapse in on itself, its outer layers rebounding against the dense inner core, and a supernova explosion will result. The blast sprays out iron at supersonic speeds, filling great swathes of space with debris that can form new solar systems. The iron in your frying pan, house keys, and blood is essentially cosmic shrapnel from the tremendous explosions that ripped through our galaxy billions of years ago. The same blasts also released carbon, nitrogen, oxygen, and other elements of life, which later produced the sun, the Earth, and eventually—you.
Curt Stager is an ecologist and climate scientist at Paul Smith’s College. He is the author of Deep Future: The Next 100,000 Years of Life On Earth, and has written for National Geographic and Fast Company. He also co-hosts a weekly science program on North Country Public Radio.