
Some marriages are arranged. Some are for love, others for convenience. Some happen as the result of a sextillion-ton collision in space. The Earth and the moon, for example.
Over the past twenty years there has been one dominant model to explain the origin of the moon: the giant impact hypothesis. The Earth is thought to have formed by a succession of collisions with moon- to Mars-sized “planetary embryos”, the building blocks of planets. The giant impact hypothesis proposes that the last of these collisions spawned the moon. A moon-sized body nicknamed “Theia”—after the moon’s mother from Greek Mythology—collided with the growing Earth. Some of the material from the impact spun into a disk of rocky debris orbiting the young Earth and, over the following thousands of years, agglomerated into the moon.
Hydrodynamical simulations have shown that the giant impact model can match the Earth and moon’s sizes and orbit. However, a key mystery remains, in the compositions of the Earth and moon.
The moon’s composition was thought to be basically indistinguishable from Earth’s mantle. Both are mostly rocky with a mixture of minerals. What’s most intriguing is that the Earth and moon were believed to be nearly identical at the isotopic level. An isotope of an element is simply that same element with a different number of neutrons Oxygen has three stable isotopes: 16O, 17O and 18O. The ratios of these different isotopes are an important chemical signature, like genetic markers. And it’s significant, historically, that the Earth and moon share an almost identical oxygen isotope fingerprint.
That similarity is problematic for the impact model, because, according to the model, the moon should have been made up of different stuff than Earth. Only ten to forty percent of the moon should have come from material that was originally part of the proto-Earth and the rest from Theia. Thus, the similarity of the moon and Earth’s footprints demands an alternate explanation.
There are at least two possible solutions to this problem. Last year, I was part of a team that proposed one. Our solution was that Theia and the proto-Earth could already have shared the same fingerprint before they collided. Our work was based on a suite of computer simulations of the late phases of the growth of the planets. We showed that the last body to collide with the growing Earth would likely be much more similar in composition to the Earth than one would expect. In ten to twenty percent of simulated collisions, the similarity was so strong that it predicted that the Earth and moon would share the same isotopic fingerprints.
A second solution proposes that the moon-forming impact was much more energetic than previously thought, generating a huge cloud of vaporized rock more than 500 times larger than Earth. According to this theory, the vaporized rock was a mixture of the proto-Earth’s mantle and Theia. The Earth’s mantle and atmosphere, and Theia’s material, became a thoroughly mixed and continuous cloud. The moon then condensed out of it.
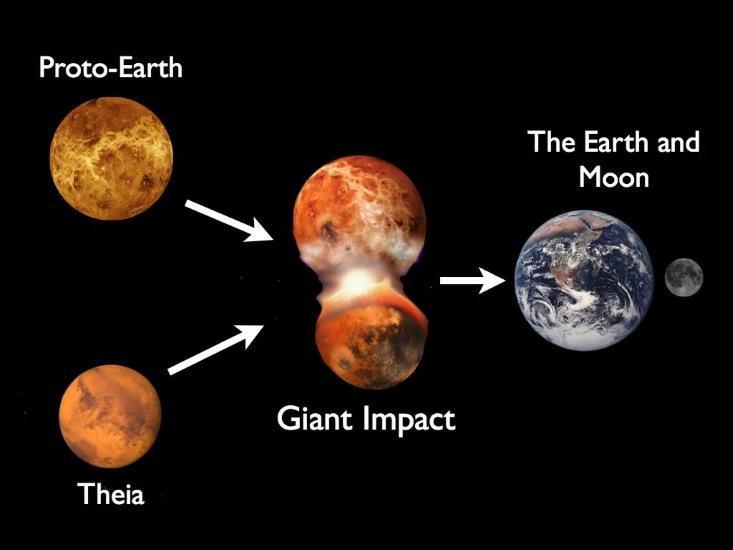
A new study in Nature by Wang and Jacobsen has recently come out in favor of the second solution. The study measured two potassium isotopes in both moon rocks and rocks from Earth’s mantle. They found that the moon rocks contained about four parts per ten thousand more of the heavy potassium isotope 41K than the lighter isotope 39K. This could be handily explained by the moon’s condensation from a hot disk of rock vapor. As the heavier isotope condenses more readily than the lighter one, and because the moon must have condensed quickly, one would expect the moon to be enriched in heavy potassium, as observed. This enrichment actually depends on the conditions within the vapor cloud, and so the measurement provides insight into the physics of moon-forming disks.
The difference in potassium isotopes between the Earth and moon is hard to explain using the first solution. If Theia and the proto-Earth had the same composition, then there would be no reason for the difference in potassium isotopes.
How does it feel to have your own scientific model fall out of favor?
It’s worth mentioning that the first solution was already on shaky ground. Another study that came out in January re-measured oxygen isotopes in moon-rocks. Our study was based on a 2014 measurement that found a difference between the Earth and moon’s oxygen fingerprints of about 12 parts per million. In our simulations of Earth’s growth, there were some examples of planets in which the last impactor matched the proto-Earth at about that level. But the new study found a closer match between the Earth and moon, with an upper bound of just 5 parts per million. The odds of matching that even-more-similar fingerprint in our simulations dropped drastically.
How does it feel, you might ask, to have your own scientific model fall out of favor? Well, I don’t feel particularly upset. Science is never based on a single idea, and often figuring out what did not happen is just as useful as figuring out what might have. It’s exciting that this new model seems to be holding together, and that we are closer to figuring out how the moon formed.
Sean Raymond is an astronomer studying the formation and evolution of planetary systems. He also blogs at planetplanet.net.






























