Early this year, Gaurav Sant will flip a switch on a machine aboard a battered barge tied up at a dock on the Los Angeles waterfront.
If all goes as expected, a pump will suck water from the Pacific Ocean through a 3-inch-wide pipe into a metal box roughly the size of a delivery van. An electrical charge flowing through that box will spark a series of chemical reactions. The water will flow out through another pipe and back into the ocean.
To the naked eye the water will look unchanged, but there will be one crucial difference: The outflow will have less carbon dioxide.
The CO2 will instead be broken into bicarbonate molecules or trapped as an ingredient in calcium carbonate, the same material corals use to build reefs. The carbon inside them will stay out of the atmosphere for millennia—and water leaving the device, now stripped of CO2, will be able to reabsorb even more of it.
Geoengineering has moved from a fringe idea to a real, albeit controversial, possibility.
On its own the machine, along with a twin project planned in Singapore, won’t make much of a dent in the planet’s greenhouse gas problem. At full throttle one of these test plants would capture 37 metric tons of CO2 in a year, the equivalent of the annual output from around eight of the cars plying LA’s legendary freeways. But if they work, Sant, an engineer at the University of California, Los Angeles, is thinking much, much bigger.
He estimates that nearly 1,800 full-size factories on coastlines around the world could coax the ocean into removing 10 gigatons—10 billion metric tons—of extra CO2 per year. That’s one-quarter of humanity’s annual carbon emissions. “These are exciting times,” says Sant.
He’s not the only one getting excited. Governments, corporations, philanthropists, and scientists are turning their eyes to the ocean as a place to stash greenhouse gas pollution.
“If you need to do something at globally relevant, economy-wide scales,” says Sant, “you have to go to the ocean.”
Several forces have converged to fuel this interest. There’s growing recognition that slashing fossil fuel use won’t be enough to meet targets in the 2016 Paris Agreement of keeping global temperature increases well below 2 degrees Celsius. Countries and companies are pledging to cut their climate pollution to “Net Zero” by mid-century. Entrepreneurs are eyeing the potential profits from selling carbon sequestration to help people make good on their promises.
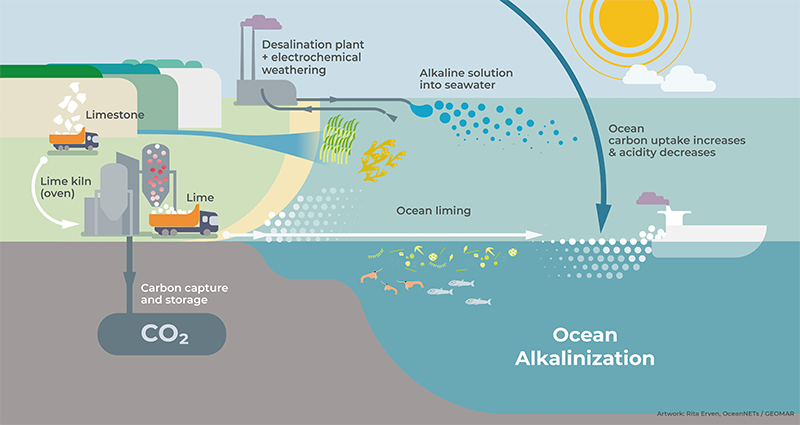
At the same time, geoengineering—large-scale environmental manipulations intended to slow climate change—has moved from a fringe idea to a real, albeit controversial, possibility. Climate engineering in the ocean gained further credibility in 2021 when the United States National Academy of Sciences, Engineering, and Medicine released a report calling for $2.5 billion in new research into approaches to sequester more carbon in the ocean.
At the moment, one of the most popular contenders is the strategy Sant is pursuing: tweaking ocean chemistry so that seawater can soak up more CO2. A Canadian startup company is preparing to pour milk of magnesia—literally—into the ocean. Much like Sant’s approach, in theory it would make the seawater more alkaline, enabling it to absorb additional CO2. The plan won a $1 million award from Elon Musk’s XPrize for carbon removal. A U.S. company is seeking the same result by spreading ground up rock on beaches; their first foray started in 2022 on a Long Island shore. Another wants to build factories that will strip CO2 from seawater in the form of hydrochloric acid.
Finally there is SeaChange, the new company Sant co-founded as a spinoff from his research at UCLA. The foundation of Facebook founder Mark Zuckerberg and his wife, Priscilla Chan, recently put $21 million into the UCLA Institute for Carbon Management, where Sant’s project began.
Despite the excitement and the funding pouring into these pursuits, much remains unknown about both how effective and economically viable any of the strategies will be, or what effects they might have on ocean ecosystems.
“It would be hard to overstate how little we know about the technologies and their potential benefits and harms,” says Lisa Suatoni, deputy director of the oceans division at the Natural Resources Defense Council, an environmental group.
The ocean is already saving us from the worst of climate change. It has absorbed 90 percent of excess heat generated by more than a century of burning fossil fuels. It is also a natural carbon sponge, holding an estimated 41,000 gigatons of carbon—45 times more than what’s in the atmosphere. By comparison, terrestrial ecosystems such as forests and grasslands hold roughly 4.8 gigatons.
While some of that ocean carbon sequestration is performed by biological sources, such as plankton, most of it occurs via chemical reactions. Seawater close to the surface soaks up CO2, maintaining a balance with nearby air. Some of that CO2 breaks down, producing carbon atoms that are passed around from one molecule to another like a soccer ball; the rest stays in the water as dissolved CO2.
Of that dissolved CO2, a portion reacts with water to make carbonic acid. Much of that acid then breaks into two smaller molecules, bicarbonate and carbonate. Each contains a single carbon atom and can drift through the ocean for millennia, altogether accounting for more than 90 percent of all the carbon sequestered in the ocean.
The potential ecological effects of altering ocean alkalinity are something of a mystery.
But as surface water gets more saturated, the ocean doesn’t absorb CO2 as readily. The buildup of carbonic acid has also made ocean water more acidic—30 percent more compared to two centuries ago. This change in pH in turn reduces its ability to break CO2 down into carbonate and bicarbonate.
In effect, Sant and the others want to give the ocean a giant dose of antacid to boost its capacity to suck up carbon dioxide.
The strategy appeals to Douglas Wallace, a chemical oceanographer at Canada’s Dalhousie University. He ticks off a number of reasons: Increasing the total store of carbon in the ocean by a tiny amount would make a huge difference in the atmosphere. It stands to reason that adding antacids would also make the ocean more alkaline, countering acidification that can make it hard for sea creatures to grow shells. And scientists have a solid understanding of the chemical reactions involved, so it should face fewer unknowns than biological strategies such as growing seaweed and sinking it to the ocean floor.
“I think it’s the only general approach that can make a difference without causing potentially massive ecosystem risks down the line,” says Wallace, who is helping to lead a United Nations-sponsored research initiative looking at how the ocean might capture more carbon emissions.
But such efforts still face major hurdles. For starters, it’s still uncertain how added alkalinity will translate into sequestered carbon. Chemistry’s pace dictates that the newly alkaline water would need to stay in contact with the atmosphere for as much as a decade to ensure that CO2 is taken up, says Wallace, and the ocean’s inevitable mixing could complicate things. Answers are critical both for understanding how overall ocean chemistry will change and to give investors confidence that when they pay for carbon to be sequestered, they’re getting their money’s worth.
Wallace hopes to get some of these answers with research he is doing in collaboration with Planetary, a Nova Scotia-based startup that plans to launch a real-world demonstration of its approach in early 2024. They will take leftover rock from a defunct asbestos mine in Quebec, extract magnesium hydroxide—an antacid that is the main component in milk of magnesia—and stir it into water flowing into the ocean from a sewage treatment plant. The company hasn’t announced the location of the experiment.
The ocean is a natural carbon sponge, holding an estimated 41,000 gigatons of carbon.
The potential ecological effects of altering ocean alkalinity are also, Wallace’s confidence notwithstanding, something of a mystery. If it makes water less acidic, there could be benefits for animals sensitive to more acidic water. Some oyster growers already boost alkalinity to seawater flowing into their hatcheries in order to increase survival of baby oysters. But there are questions about downsides, says Adam Subhas, a chemical oceanographer studying ocean alkalinity at the Woods Hole Oceanographic Institution.
Alkaline rock spread on beaches or dumped into the ocean might be tainted with toxic metals such as nickel. Traces of iron and silicate could spark algae blooms which can cause oxygen levels in the water to fall. Altering the acidity of water could also cause subtle shifts in complex food webs, such as giving an edge to shell-creating plankton called coccolithophores over diatoms, a type of plankton that doesn’t make shells. “Is that going to cascade through the food web in a way that might change the whole way the ecosystem functions?” muses Subhas.
Ken Buesseler, also an oceanographer and biochemist at Woods Hole, fears such questions are being neglected amidst the rush of companies eager to tap into fledgling carbon markets.
He has seen firsthand what can happen when hype and the pursuit of profit outrun the science. For decades Buesseler studied how iron and other elements can fuel algae blooms that can help sink carbon dioxide deep into the ocean. In the early 2000s, interest in the strategy surged—but backlash followed among environmentalists concerned about poorly controlled experiments. In 2012 one company mounted a secretive expedition that dumped 110 tons of iron into the ocean off the northern coast of British Columbia. The ensuing furor halted most serious research in the field.
Buesseler warns that newly popular approaches, including ocean alkalinity enhancement, could be on a similar trajectory. “I really worry that we think we’ve got the single shiny object that’s on this curve where we don’t know a lot about it, so it’s cool and investment flows in,” he says. “Then they’ll be where iron was. It won’t be easy to quantify the benefit. They won’t have studied the ecological impacts. And it won’t scale as readily. And that will shut down potentially legitimate carbon dioxide removal work.”
Even if scientists find that tinkering with ocean chemistry is benign and effective, it—along with any other strategy—faces more major hurdles. It needs to be economically viable. And it needs to grow big enough to match the problem.
Sant has spelled out the staggering scale of what might be needed. In a 2021 paper in the journal Sustainable Chemistry & Engineering, he and his colleagues estimate that removing 10 gigatons of CO2 would require pumping 3.5 trillion tons of water—roughly equal to the water withdrawn each year around the world from every aquifer, lake, river, and the ocean. Some 1,760 new factories, each the size of modern sewage treatment plants, would need to be built, and powering them is another issue: It would take more than 20 petawatt hours of electricity per year, equal to roughly 70 percent of the world’s total electricity production in 2021.
While the numbers are boggling, Sant seems undaunted. He sees a parallel in the trillions of dollars spent by the U.S. alone in responding to the COVID-19 pandemic. “This is the kind of money that you’re talking about,” he says. “A couple of percent of global GDP that needs to be deployed to be able to address this at scale.”
For the moment, though, he is focused on something much smaller: getting his machinery up and running at the docks in Los Angeles. If all goes well, it will be a first, very tiny step toward addressing a planet-sized problem. ![]()
Lead illustration by GoodStudio and BigMouse / Shutterstock