One day in 2010, when oncologist Paul Muizelaar operated on a patient with glioblastoma—a brain tumor infamous for its deathly toll—he did something shocking. First, he cut the skull open and carved out as much of the tumor as he could. But before he replaced the piece of skull to close the wound, he soaked it in a solution containing Enterobacter aerogenes,1 bacteria found in feces. For the next month, the patient lay in a coma in an intensive care unit battling the bacteria he was infected with—and then one day a scan of his brain no longer showed the distinctive signature of glioblastoma. Instead, it showed an abscess, which, given the situation, Muizelaar deemed a positive development. “A brain abscess can be treated, a glioblastoma cannot,” he later told the New Yorker. Trying it, he thought, was worth the chance. He had done this only as a means of last resort in a couple of hopeless cases—but ultimately, his patients still passed away, which led to a scandal that forced him to retire.
Muizelaar’s approach may sound beyond outrageous, but it wasn’t entirely crazy. For over 200 years medics have known that infections, particularly those accompanied by fevers, can have a strange and shocking effect on cancers: Sometimes they wipe the tumors out. The empirical evidence for these hard-to-believe cures has been documented in medical literature, dating back to the 1700s. In the 19th century, some doctors tried treating cancer patients by deliberately infecting them with live bacterial pathogens. Sometimes it worked, sometimes the patients died. Injecting people with dead bacteria worked better and, in fact, saved lives, at least in some cancers. The problem was that it didn’t work consistently and repeatedly so it never became an established treatment paradigm. Moreover, no one could explain how the method worked and what it did. Doctors speculated that infections somehow revved up the body’s defenses, but even in the early 20th century, they had no means of elucidating the mysterious force that devoured the tumor.
Today we know that this mystery lies in the complex interplay of cancers and the immune system, says Mikala Egeblad at Cold Spring Harbor Laboratory, who studies the tumultuous interactions between cancers and the organisms they grow in. We know that cancers have an uncanny ability to pull wool over the eyes of the immune system’s cells—not only by hiding from them, but even co-opting them to help themselves flourish. “Tumors are dysregulated organs,” says Egeblad—and they dysregulate the environment around them too. They cause a lot of turmoil and havoc wherever they take hold. Called the tumor microenvironment, that “battleground” is teeming with various microscopic players that cancers corrupt into unwitting allies.
“Our immune system is trying to protect us from various threats, including cancer,” says Karin Pelka at Gladstone-UCSF Institute of Genomic Immunology who studies the cellular interactions that shape immune responses. “But cancer mutates in ways that it evolves to evade the immune system. So there’s a constant battle going on.”
In these dysregulated, messy ecosystems, infections may indeed serve as a force that rights the wrongs. They could reboot the body’s normal defense mechanisms, making the immune system see the enemy. However, deliberately infecting cancer patients with bacterial pathogens faces a major obstacle. It will never pass FDA approval because subjecting people—who are already gravely ill and fighting for their lives—to yet another health threat is unethical, reckless, and risky. And yet new directions in cancer treatment draw on the immune system kickstart idea, albeit in a different way. Moreover, some of the immunological approaches to cancer treatments have graduated from clinical trials to actual clinics.
He had done this only as a means of last resort in a couple of hopeless cases.
Today, medicine has better methods for resetting the body’s idle defenses that don’t involve infecting patients with pathogens. And there are different ways to do it, says Pelka. One of them employs the so-called oncolytic viruses—genetically engineered or naturally existing viruses that infect only tumor cells, multiply inside, then burst them open, invading more cells. Scientists are also trying to boost the immune system activity with specific cytokines—molecules that cells use to communicate with each other. An especially successful class of drugs now used against different types of cancers is called checkpoint inhibitors; it works by unleashing the body’s warrior T-cells to kill cancer cells. Another strategy that has proven successful against some blood cancers are CAR-Ts (Chimeric Antigen Receptor T-cells), in which T-cells are taken from the blood, engineered in a lab to seek out the specific cancer—and then infused into the patient.
Revving up the immune system defenses is also much gentler on patients than the traditional methods like chemotherapy, which inevitably damage healthy cells, too. “The immunotherapy is much less toxic than chemotherapy,” says oncologist Sylvia Adams, who treats cancer patients at NYU Langone Health System. “It doesn’t change the patient’ quality of life.” It just “coaches” the immune system to tackle the tumor. And that’s what oncologists are aiming to tap into.
We hope to train the immune system to recognize the tumor,” Pelka says. This training could have a long-lasting effect because the immune system has a memory. Once the chemo is stopped, the cancer can regrow if not every single cell is killed. But if you train the immune cells to recognize the enemy, they will remember it. “This memory function is something that cancer immunologists are very excited about,” Pelka says.
This immune system “training” works on the molecular level, and that’s what cancer immunologists are investigating right now. Egeblad does it in a Fantastic Voyage style—by watching what the cells in the tumor microenvironment do. It is a bit like parachuting into the tumor trenches where the armies of cellular soldiers engage in military actions, sometimes deceiving each other, sometimes waking each other up from their molecular stupor. Egeblad is experimenting with a once-promising treatment that had fallen into disfavor because it also involved dangerous bacterial pathogens. First tried by a clever clinician over 100 years ago, it may be finally due for its 21st-century upgrade.
William Coley and his Toxins
In the fall of 1890, Elisabeth Dashiell, an athletic 17-year-old lady who was a close friend of John D. Rockefeller, Jr., came back from an adventurous trip to Alaska with a swollen hand, which she had hurt in a seemingly minor accident. Her hand was healing so poorly that she went to see William Coley, a young but prominent doctor, at the Memorial Hospital in New York, which later would become the Memorial Sloan-Kettering Cancer Center.2
It turned out Dashiell’s hand wasn’t healing at all—Coley diagnosed her with an aggressive round cell sarcoma, a type of bone cancer.3 Coley operated, but it did little to help—Dashiell died from the metastases 10 weeks later. Her cancer was so rapid and vicious that it left a profound impression on Coley. He embarked on a quest for better options.
While scouting medical literature, Coley found the seven-years-old medical records of a patient who had round cell sarcoma on his neck, which kept growing back despite five surgeries. The man, a German immigrant named Fred Stein, was considered inoperable and hopeless, until he contacted erysipelas—a skin infection caused by streptococcal bacteria that manifests itself in fever and large, red patches on the face and legs. The infection, which spread over his neck and face, produced a strange side effect—his tumor all but vanished. According to the records, Stein went home in good health. Coley searched the Lower Manhattan tenements for Stein, and found him still alive and well, with no signs of cancer.
As he continued plowing through medical literature, he found that various prominent medics also had observed curative effects of infections on cancer. For example, English surgeon Sir James Paget noted that infection may cause a regression in some tumors. In 1867, German physician Busch reported a case similar to Stein’s, in which a tumor disappeared when the patient developed erysipelas. And in 1888, only two years before Dashiell died, another medic named Bruns intentionally gave a cancer patient a shot of streptococcus to induce erysipelas, after which the tumor shrunk. Altogether, Coley read about over 40 cases documenting the beneficial effects of infections on tumors.
The teams showed the combo suppresses tumor growth and metastasis.
Coley tried injecting three patients with streptococcal bacteria. The tumors seemed to shrink, but two of the patients died from the infection, so Coley switched to using dead bacteria—killed by heat. He also added another “cooked” bacteria into his concoction, Serratia marcescens, which, when alive, can cause infections of the respiratory and urinary tracts. He used the combo, which was dubbed Coley’s Toxin(s), on inoperable sarcoma patients with a fair amount of success—it was better than anything else available at the time. For the next three decades, Coley’s Toxins were widely used—until radiation and chemotherapy techniques came of age. These newer treatments soon surpassed the dead bacteria in popularity and Coley’s Toxins were all but buried in the annals of medicine.
Coley’s Toxins had several problems. Medics like predictable and repeatable results, and the bugs—alive or dead—were finicky subjects. Coley made 13 different preparations of the toxins, with some more effective than others. Sometimes he administered them intravenously, sometimes intramuscularly and in other cases he injected them directly into the tumors.2 Many doctors who used his toxins didn’t get the same results. Moreover, no one, not even Coley, could elucidate how the toxins worked.
Part of the issue was that Coley’s method was essentially ahead of its time. In the early 20th century scientists didn’t have the means to take a Fantastic Voyage trip into the tumors’ den. They couldn’t peek at the tumor microenvironment. They didn’t know that cancers can corrupt and co-opt the immune system cells. And yet, Coley was on the right track, Adams says. “When we, oncologists, talk about cancer immunology, we always refer to Coley as the person who had the first inkling into the power of the immune system.”
Today, scientists have much better tools to watch these battles in action. They can literally see the toxins flipping the immune cells’ tumor-tackling switches back on.
Into the Tumor’s Trenches
If you could indeed journey into the tumor trenches, you’d likely find the place very crowded. Tumors like to surround themselves with all kinds of normal, healthy cells, which they corrupt and co-opt into helpers.
In a healthy environment these cells would be performing their designated activities, Egeblad explains. Fibroblasts would be building scaffolding for various tissues to grow, such as muscle or bone. Pericytes would be making blood vessels. The immune system warriors B-cells and T-cells would be scouting for perpetrators, releasing antibodies, and killing the sickly cells—those infected by pathogens or mutated. Neutrophils would join the fight by ingesting invading microorganisms and releasing enzymes that kill them. And macrophages would clean up all the cellular debris and zap various rogue cells with nitric oxide—a toxic, free radical molecule they spew out. Many of these cells also interact with each other through molecular messaging. T-cells stimulate B-cells to secrete more antibodies. Macrophages activate T-cells to sic them on the agents of disease. In response, T-cells activate macrophages by spitting out inflammatory cytokines, molecules that regulate the body’s response to disease and infection. All these different players keep each other alert and engaged, a well-working biological defense team.
He injected three patients with streptococcal bacteria. The tumors seemed to shrink.
Normally, all these activities are supposed to spot mutated cells and wipe them out before they proliferate. But if and when a mutated cancerous cell—which may divide into two, or four, or a little clump—manages to avoid detection, they break the normal order of things. They start issuing their own molecular messages that confuse the cellular team. Tumors corrupt fibroblasts, which, in turn, turn off some of the T-cells and B-cells, essentially making them blind to the cancers’ presence. Tumors can “reprogram” macrophages—they secrete molecules that attract these cells, but instead of devouring the mutants, macrophages release growth stimulants for them. “So the immune system can provide the tumors with growth factors, which benefit the cancer,” says Pelka.
Scientists call such molecular “turncoats” tumor-associated macrophages, or TAMs. These TAMs do more damage than just feeding the tumors—they turn off T-cells and B-cells, so they no longer see the enemy. Moreover, these TAMs start taking cancer around the body, enabling metastases to take hold. They actively help malignant cells hitch rides in the bloodstream, traveling far and wide and settling in new locations. “Data strongly suggests that TAMs help tumor cells in and out of blood vessels—they are physically nurturing cancer cells,” says Egeblad. “So even though the immune system has the ability to recognize the cancer cells, it gets turned off. The cells get suppressed.” Cancers indeed pull the wool over the immune system’s cellular army. The cells need an eye-opener.
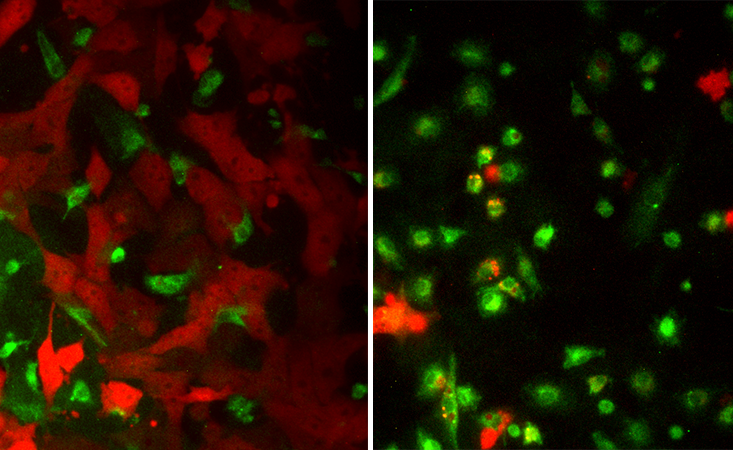
For Egeblad such an “eye-opener” was an experiment one of her post-doctoral researchers did a few years ago. He was trying to make TAMs go back to their normal feisty state and start killing glioblastoma. He mixed a bunch of cancer cells and TAMs in a petri dish, and then he added some “magic dust”—a mix of bacteria-derived toxin and another immune-boosting compound called interferon gamma. A part of the innate immune system, interferons are proteins that mediate the body’s defense responses, and the gamma type is specifically known for its anti-cancer activity.
The toxin-interferon combo packed a punch. The blinded macrophages “woke up” and attacked cancer—a battle that the post-doc captured on camera. “It makes macrophages speak in a different way to the T-cells,” explains Egeblad. “They change the signals they are sending out, and these new signals make T–cells effective in recognizing the cancer cells. But we think it also likely works on all other cells, too. It changes the entire environment.”
Egeblad and Adams teamed up to investigate how this combo would work on tumor cells taken from real patients. Adam’s team collected lung fluids from patients with breast cancer that had metastasized to the lungs and transported them to CSHL. Egeblad’s team extracted tumor cells and immune cells from the samples, treated them with the toxin-interferon combo and watched the immune cells waking up to the cancer’s presence. “We were able to turn them on to the tumor cells in the dish,” Adams says. “We reprogrammed them to kill the tumor.” In a recent study, the two teams showed that the toxin-interferon combo also suppresses tumor growth and metastasis in breast and ovarian cancer in mice.4 They hope to eventually try this in humans, too.
Understanding the tumor microenvironment has other potentials. It might help answer exactly how cancers first “set up shop,” corrupting immune system cells and making them work for themselves. When metastatic cancers first arrive to a new location, that location isn’t set up to nourish them, Egeblad says. All the body’s cells there are healthy and doing their regular job—and yet, the cancer manages to corrupt them again. Too often patients go home seemingly cured from their cancers, only to discover that it metastasized someplace else, or even many places, and is already in advanced form. “We’d like to understand how metastases develop, what enables cancer cells to succeed in the new organ or how it gets eliminated by the immune system there,” Egeblad says. “Once we figure that out, we’ll be able to put an end to cancers’ spread.”
Video One: Troubled Waters In this video, the macrophages are unable to destroy cancer cells. The breast cancer cells grow and eventually take over. Credit: Cold Spring Harbor Laboratory
Video Two: New Hope In this video, researchers in CSHL Professor Mikala Egeblad’s lab used drugs to activate the macrophages. With that boost, the macrophages are able to destroy the cancer cells. Credit: Cold Spring Harbor Laboratory
Lead image: SciePro / Shutterstock
References
1. Eakin, E. Bacteria on the brain. The New Yorker 2015.
2. McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. The Iowa Orthopaedic Journal 26, 154-158 (2006).
3. Kienle, G.S. Fever in cancer treatment: Coley’s therapy and epidemiologic observations. Global Advances in Health and Medicine 1, 92-100 (2012).
4. Sun, L., et al. Activating a collaborative innate-adaptive immune response to control metastasis. Cancer Cell 39, 1361-1374 (2021).