Twenty years ago, Bill Jacobs made the tuberculosis bug glow. It was like mounting a pair of headlights on a man-eating tiger. One of the world’s deadliest infectious diseases could no longer slink around in the shadows, evading its trackers. Microbiologists could now peer into a microscope to see if a particular antibiotic turned out the lights—that is, killed the TB bacteria. Or at least they could do this so long as they didn’t happen to be Bill Jacobs. That’s because when Bill Jacobs looked into the microscope at his own creation, he couldn’t see a thing. He was going blind.
When Jacobs looks at me today, first he sees my left eyeball, then he sees my nose, then, my lips. He sees the world in pieces. One. Piece. At a time. Imagine rolling up a magazine and holding it to your eye. That’s what Bill Jacobs sees. His field of vision is a pinhole of clarity no more than a few inches wide and shaped like an amoeba. A genetic disease called retinitis pigmentosa has long since destroyed the rod photoreceptors in his eyes, which is why he can’t see under the dim light of a microscope, and it is now eating away at last of his color-sensing cones.
When I look at Jacobs, I see a man in a sage-colored sweater with snowy white hair hunched over his desk, squinting and pecking at a canary yellow keyboard with oversized letters on it. We’re inside his laboratory at the Albert Einstein College of Medicine in the Bronx, and I watch Jacobs as he moves the mouse pointer to the upper left corner of the screen so that he can find it in his tunnel of vision. Then, he clicks on a scientific paper and listens as a female voice comes out of his computer speakers, enunciating scientific terms and abbreviations with all the panache of C-3P0. It’s hard to believe that this is how one of the world’s premier TB investigators gets his work done. “It is what it is,” he says in his charmingly low-key way. “At least, I can hear.”
The son of a steel worker in Pittsburgh, Jacobs thought he would be an astronomer gazing up at the night sky. His mother thought he needed to look where he was going. “Look down! Look down!” she would yell. He’d bump into the coffee table, bruising his shins or knocking over drinks. When his mother took him to the ophthalmologist, he examined the back of his eye with a special instrument and could see that Jacobs’ retina looked inflamed. But there wasn’t anything science could do for him. This was in the 1950s.
Jacobs decided to take his mother’s advice and start looking down. He was drawn to the abstract challenges of mathematics, but he was also passionate about the underdogs of the developing world and discovered that bacterial genetics was, what he calls, “the mathematics of biology.” In graduate school at the University of Alabama at Birmingham, he studied the genetics of leprosy, which is caused by a bacteria in the same family as TB, the mycobacteria. However, leprosy mycobacteria can’t be easily grown in laboratory mice. Instead, Jacobs and his colleagues took biopsies from people in leper colonies and stuck them into nine-banded armadillos, an animal whose eyesight just happens to be as bad as his own. It took two years before he could collect data. His friends thought he was either crazy or stupid—at least until he started publishing a string of high-profile papers. “You follow what feels right in your heart,” Jacobs says. After that success, he figured cracking the genetics of TB would be a breeze.
The bacterium behind TB, Mycobacterium tuberculosis, kills more than a million people every year. It typically attacks the lungs first, which leads to a bloody cough, but it can also spread to other parts of the body, such as the brain and the bones. Even with the proper antibiotics, an infection requires between six and nine months to clear completely because TB can hunker down and wait the drugs out. And for some people treatment takes even longer because drug resistance is spreading.
TB has proven particularly devastating among people with HIV who have a compromised immune system. They often fail to complete treatment because of the unpleasant, and sometimes lethal, side effects of TB drugs when mixed with antiretroviral therapy. In 2006, the world learned just how bad the TB crisis had become in South Africa, when the disease killed 52 HIV-positive people within a few months at a hospital in the remote town of Tugela Ferry. The four TB drugs doctors tried to treat them with had all failed.
However, long before the Tugela Ferry outbreak—back in the late 1980s—Bill Jacobs predicted that new tools to fight TB would be needed. He realized that the only way to develop drugs, vaccines, and diagnostics was to find a way inside the then-impenetrable M. tuberculosis genome. To enter the microbe, Jacobs thought about a group of bacteria-infecting viruses, called phages, that might hijack the machinery inside the TB cell. But he wasn’t sure exactly how to put phages to use in his research. Then, early one morning while he was lying in bed in the dark, he had an epiphany. “It was almost like a waterfall of ideas,” he says. “I’d been thinking for a month and—Boom!—it hit me.” He had figured out a trick to insert genes into a special region of the phages’ genome, which would shuttle them into the TB bug and help him perform all kinds of experiments.
Soon after Jacobs reported the technique, a couple of scientists from the British medical diagnostics company Amersham told him about a gene from fireflies, called luciferase, that could make salmonella light up. They proposed shuttling luciferase into TB to create a simple diagnostic test. At the time, a TB diagnosis required at least a month. The collaboration with Amersham never happened, but Jacobs followed up on their suggestion. He realized that the more immediate benefit of glowing mycobacteria would be to researchers studying TB drug resistance and susceptibility. His team successfully illuminated the bug, but Jacobs only learned about the achievement secondhand. ”Everybody else could see it in the dark room,” he says. “I could never see it with my own eyes.”
Fast-forward a couple of decades: One summer day in 2010, Paras Jain, a researcher in Jacobs’ lab, invited his boss to see the new generation of phages the team had created. Rather than using the firefly gene, they had found a way to shuttle green fluorescent protein, which comes from a jellyfish, into their phages. Jacobs followed Jain down the hall and through the door of the imaging room, where a sign warned “Live Cell Imaging in Progress.” Jain told Jacobs to look into the microscope, and Jacobs was astonished. “I see it! I see it! I see it!” he shouted. The new gene had boosted the light levels in TB by a factor of about 100.
After Jacobs returned from a trip to South Africa where he struggled to give a lecture in a dark room, he gave Stone a call. “We’ve got to do something,” he told him.
So far, Jacobs’ glowing phages have not transformed diagnostics in the field, in part because they require more infrastructure than is available in clinics in the developing world. One commercially available phage-based diagnostic, called FastPlaque, detects resistance to the antibiotic rifampicin, but it has gotten mixed reviews in real world trials. Still, Jacobs believes his glowing phages hold the promise of being the ideal diagnostic one day, and in the hands of researchers, they continue to reveal many of TB’s secrets.
Last year, for instance, Jacobs was trying to crack the enigma of why TB takes so long to treat. If you add the right antibiotics to a test tube, 99 percent of TB cells die within a week. But there are always a few TB cells that survive, about one in a thousand. They seem to switch on a different genetic program to make them grow more slowly than normal, like hardy desert seeds holding out for better conditions. Jacobs and his colleagues designed a new phage that would make these persisters glow red rather than green. This allows them to spot these persisters early on, automatically sort them, and follow their reaction to different drugs during experiments. By chance, Jacobs discovered that the persisters were sensitive to oxidative stress, which causes DNA damage. To test his theory, he added a dose of vitamin C to a strain of TB that can withstand four different antibioitics. Vitamin C, which can cause oxidative stress, wiped the persisters out in just four weeks.
“Are you serious?” Jacobs’ friend Ian Orme asked. Jacobs said he was. Orme, an immunologist at Colorado State University, decided to test vitamin C on a few different strains of TB that he keeps at his lab. “Lo and behold, Jacobs was 100 percent correct,” Orme says. Jacobs is now testing the effects of Vitamin C on TB-infected mice in his lab. The amount of Vitamin C required is substantial, and Jacobs does not know whether adding that much Vitamin C to a person’s diet is a safe or realistic treatment. Rather, he believes, researchers might start designing drugs to target this fresh Achilles’ heel.
Jacobs has become so successful in his field that some of his colleagues don’t realize how bad his vision is getting. Every day, he sees less and less of the world. A fog is closing in, and his vision now goes completely black if he moves from a well-lit room to a poorly lit one. He’s finally considering getting a guide dog. He never thought he had a good memory, but he has developed an exceptional ability to listen to scientists share their results at meetings. “You tell me something, I won’t forget it,” he says.
Somehow, his fading sight hasn’t slowed him down. He has published over 300 papers and been issued 25 patents. What’s his secret? He’s basically fearless, says Graham Hatfull, a collaborator who travels to South Africa with him every year. A while back, they went on safari, and Hatfull was white-knuckling it at the wheel. “You have to get close enough to the elephant so that Bill will be able to see it,” he says. “The way that Bill works in general is he’s not shy about taking on ambitious projects.”
Since 1988, Jacobs has been a fellow with the Howard Hughes Medical Institute, which funds his research. During one of the annual meetings, he met another fellow, an ophthalmologist named Edwin Stone, who was working on cures for blindness. Stone had spent the last 25 years collecting DNA from people like Jacobs who had retinitis pigmentosa, and Jacobs was always fascinated by his talks. A few years ago, after Jacobs returned from a trip to South Africa where he struggled to give a lecture in a dark room, he gave Stone a call. “We’ve got to do something,” he told him.
Stone told Jacobs to come to his lab at the University of Iowa, where he was working on a new project. Stone sequenced part of Jacobs’ genome and compared it to Jacobs’ sisters. He found that Jacobs had a recessive mutation on a gene called SNRNP200, which is involved in gene splicing and likely caused his condition. Stone determined that this mutation was a blip present in fewer than one out of every million people.
Recently, Stone took cells from Jacobs’ arm and transformed them into a kind of cellular Silly Putty known as pluripotent stem cells. Pluripotent stem cells can turn into just about anything: a liver cell, a blood cell, a neuron. Naturally, Stone is turning them into photoreceptors. He hopes to fix the blip in their genes and then put them back into Jacobs’ retinas to replace his lost rods and cones. He’s coy about predicting how long it will be before Jacobs can see again, but he hopes it will be less than a decade.
Now, Bill Jacobs, who had spent his entire life as a researcher, is suddenly a research subject. “People have always asked me in the past ‘Why did you get into science?’ and it was never because of my eyes,” he says. “Now, to imagine we could actually find this mutation and do something that might benefit me is very cool.” At the same time, he’s realistic about the prospects for success. “I may not get a cure but the knowledge we are going to get from it will benefit somebody down the road,” he says. He pauses, and then adds: “If I get a cure, it’s gravy.”
In the meantime, Jacobs continues to push his boundaries. In his office, he rarely uses a cane and navigates around on instinct, moving more quickly than caution would recommend. At one point I think he’s shoving me to get out of the way, but I realize I had slipped outside his field of vision. He grabs me and points out the window to the porches of a now-vacant sanatorium next door: a relic from a time when doctors thought fresh air and sunlight helped cure TB. When that didn’t work, they’d remove chunks of patients’ lungs.
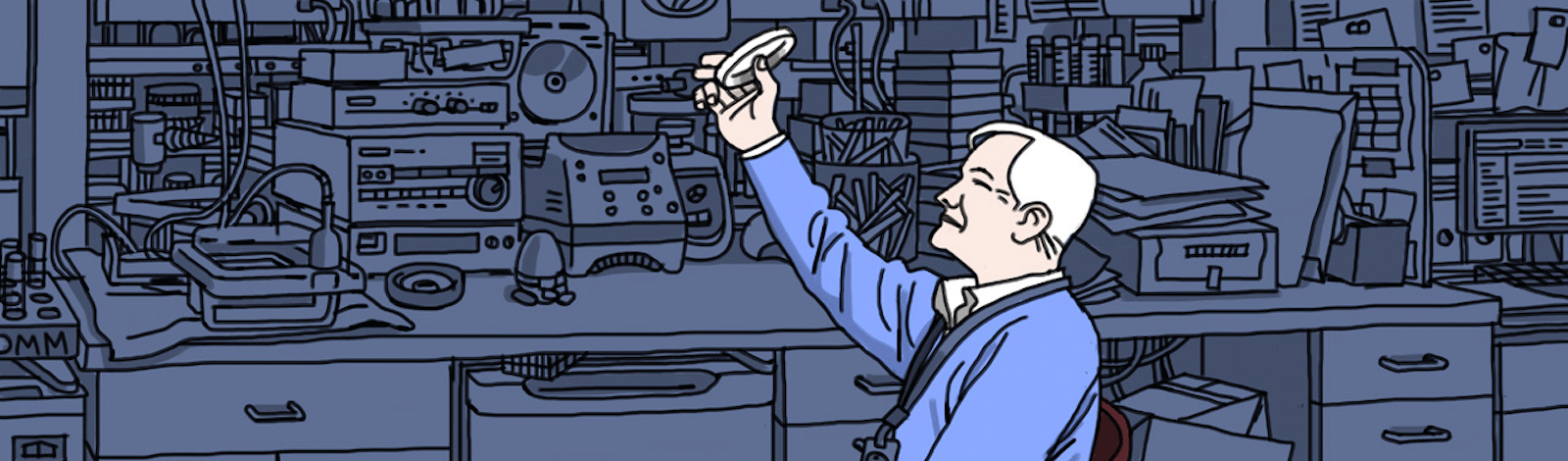
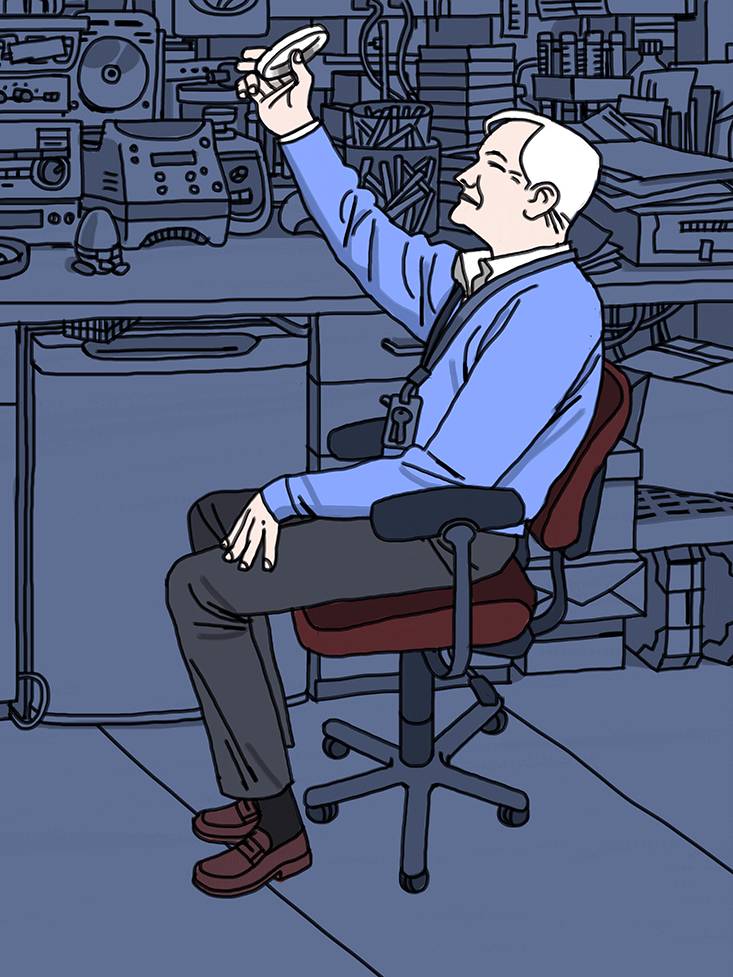
Once we’re in his lab, Jacobs calls over a few members of his team, and they bring him a stack of petri dishes wrapped in aluminum foil. The other phages that Jacobs has developed insert themselves into any bacteria in the mycobacteria family, but the phages in these dishes target the TB bug specifically. Like his other creations, this new phage has been designed to deliver the green fluorescent protein, known as GFP. “We’ve been dreaming of making the GFP version of this for a year now,” he says. He takes a step back and holds one of the dishes up to his right eye in awe. It’s dotted with hundreds of tiny plaques, spots where potent phages have killed off the bacteria. As he counted up the plaques, we all stood there looking at him. There’s no other way to put it. He was glowing.
Brendan Borrell is a freelance journalist based in Brooklyn.