For many people, 1969 felt like a year when technology could solve all of our problems, a sentiment that reached a crescendo with the Apollo 11 moon landing. But back on Earth, Michael Kaback was a faculty member in pediatrics1 at Johns Hopkins Medical Center, and he was frustrated. Kaback had helped some of his colleagues to diagnose two young children with some alarming symptoms: They started having seizures, stopped developing mentally, and were growing weaker, letting their limbs flop from side to side. Since the parents of both children were Ashkenazi Jews, Kaback suspected that the cause of the disease might be genetic. So he checked the children’s blood samples for a recently discovered enzyme called hex A—without it, cells in the central nervous system can’t break down an essential fatty substance, causing the cells to deteriorate and die over time. Both children were missing the enzyme, which confirmed Kaback’s fear: They had Tay–Sachs disease. They would die before they turned 5 years old, and there was nothing anyone could do.
From these two families, Kaback got a sense of how devastating this disease can be for families. He also realized that he had the technology and knowledge to start screening people for the Tay–Sachs gene before prospective parents contemplated having a child. It would’ve been hard to predict back then, but Kaback and his collaborators embarked on a massive public-education campaign about genetic disease, starting in the Baltimore-Washington, D.C. area and eventually spreading across the country and the world. They successfully navigated an ethical minefield and helped nearly eradicate a fatal genetic disease—and established as a model for screening of myriad genetic diseases to come.
Tay–Sachs qualifies as a rare genetic disease; in the 1970s, about 1 in 3,600 children born to an Ashkenazi Jewish family had it. (The most convincing theory as to why the disease disproportionately affected Ashkenazis is that, back in Eastern Europe, populations of Jews were kept isolated and mostly married within their own community, causing the mutation to become more common.)
Tay–Sachs is a recessive trait, so there are healthy people who carry one version of the disease gene and never have any symptoms—they only learn that they are carriers if they take a genetic test. (The disease only manifests if someone has two Tay–Sachs genes.) Understanding how the disease is passed down is surprisingly simple—it works the same way as Gregor Mendel’s pea plants, which you might remember putting into a Punnett square in middle or high-school biology class.
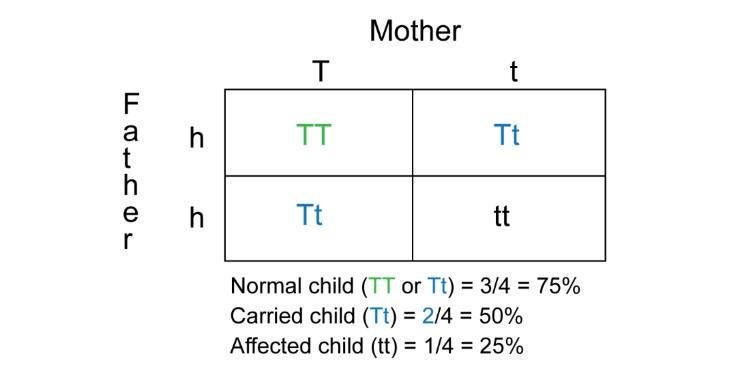
Each parent in the diagram has two different alleles, or versions of the gene: one dominant, healthy version and one recessive version that causes the disease in anyone who has two copies of it. In the Punnett square, three of the four offspring have at least one healthy allele: two of them are carriers, with one healthy and one disease gene, and one has two healthy genes. But the offspring in the bottom right of the square receives two disease genes, which means that child will have the disease. Should two carriers have a child, that child has a 25 percent chance of getting two Tay–Sachs genes, bringing the disease with it.
When Kaback started the program2, if the two parents were found to be carriers, they could still have a natural-born child if they elected to monitor each pregnancy through amniocentesis, where a small amount of fluid is removed from the amniotic sac and tested, starting from 14 weeks of pregnancy. If the baby was found to have Tay-Sachs, the mother could terminate the pregnancy. Later on, a technique called chorionic villus sampling, which tests a sample of the placenta, allowed them to determine if the fetus had the disease at just 10 weeks of pregnancy. With today’s reproductive technology, if the two parents are found to be carriers, it’s easier to safely have a child: An egg is fertilized in the lab using IVF and then genetically tested. If it’s deemed disease-free, it’s implanted in the mother’s uterus. Any embryos found to have the disease are destroyed.
Because Tay–Sachs was more frequent in a specific population and was so objectively severe, it was a good first candidate to be addressed through widespread genetic testing. “When you look into the literature, there’s an agreement that Tay–Sachs was a relatively simple [ethical] problem, compared to tests offered today or in the future,” says Julia Inthorn, a medical ethicist at Göttingen University in Germany. Community members and medical professionals agreed that it destroyed families, and there wasn’t (and still isn’t) any cure or treatment.
But before the carrier screening could become commonplace, there were still a number of ethical concerns that had to be addressed. “Some people in my own profession raised questions about stigmatizing Jews—they said, ‘Why do we have to bring this up?’” Kaback says. Others worried about what couples would do with the information, like pursue late-term abortions. Conservative Jewish communities were opposed to the embryo-killing aspect of in vitro fertilization.
Kaback and his collaborators were able to work through these ethical issues and get unprecedented community participation through what Kaback sees as three integral components: community education, voluntary screening, and genetic counseling. He started by educating community leaders, volunteers, and physicians that serve Jewish communities about the genetics behind the rare disease. “The idea was knowledge—if people had it and could communicate it, they would make decisions in their best interest,” Kaback says. Then they started offering voluntary screenings, held at synagogues and community centers, for married couples over the age of 18, then later allowed single people to get screened, too. Samples were “blinded,” sent to laboratories with an identification number and without a name in order to prevent patients from being stigmatized. Families with positive results would sit down with a genetic counselor about what the results meant and their reproductive options. Since this structure worked in the Baltimore–Washington area, Kaback started bringing it to other U.S. cities with sizable Jewish populations—New York, Los Angeles, Chicago—and eventually to others around the world. By 1993, similar programs had sprung up in 15 countries on six continents.
“From a technology standpoint, we can screen for 150 conditions. But at what point do we decide what conditions we should be screening for because of the severity of the implications?”
The program worked: By 2001, the prevalence of Tay–Sachs was reduced by 90 percent, from 50 cases per year in the U.S. to five. Today, remarkably, it’s more common in non-Jewish families than among Jews.
Working with religious leaders was essential in getting good uptake in the community, but Kaback was adamant that no individual organization, religious or otherwise, should run the testing program; “it has to have a medical genetics leadership,” he says. The same structure has been used around the Mediterranean to screen for beta thalassemia, a genetic disorder of varying severity that affects the blood; before marrying a couple, the Orthodox church of Cyprus requires a certificate showing that both people have been screened for beta thalassemia.
In many ways, Kaback and his collaborators paved the way for other genetic screening programs. “Tay–Sachs is often used as a positive example and proof to show that reduced prevalence of the disease is really possible,” says Inthorn, the medical ethicist. “For the ethical debate, the communities had to solve all those questions themselves and they did it brilliantly—and they published it, which helped ethicists later on.”
But many of the ethical questions have become thornier in recent years, as carrier screenings for Tay–Sachs are commonplace and often combined with tests for a rapidly growing list of other genetic markers. “From a technology standpoint, we can screen for 150 conditions. But at what point do we decide what conditions we should be screening for because of the severity of the implications?” says Jennifer Hoskovec, a professor at the University of Texas Medical School who specializes in prenatal genetics. Having a disease-related gene doesn’t always mean a person will get that particular disease, and many diseases don’t diminish a person’s life to such an extent that it should end before it begins. Should you choose not to implant an embryo because it has Down syndrome, or is intersex, or has the BRCA breast cancer gene and will likely develop breast cancer, when the individual could have a perfectly lovely life anyway?
These decisions aren’t as cut-and-dry as with Tay–Sachs disease, and there’s not just one answer. “Now we’re having more open conversations about the fact that there are decisions out there,” says Hoskoveec. “Parents have to take [genetic] information and make decisions that are best for them.”
Corrections:
1. The article originally said that Michael Kaback was a medical resident in 1969 when he was working on Tay–Sachs.
2. The article was changed to clarify that IVF and pre-implantation genetic diagnosis were not available in the 1970s.
Alexandra (Alex) is a science writer. Currently based in New York City, she has previously lived in Brattleboro, Vermont; Lima, Peru; and Washington, D.C. She tweets at @alexandraossola.